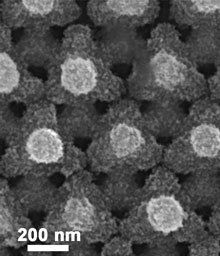
Foam power: This lithium-ion battery cathode can be used to make a battery that holds as much energy as a conventional one, but can recharge a hundred times faster.
Credit: Paul Braun
A new way of making battery electrodes based on nanostructured metal foams has been used to make a lithium-ion battery that can be 90 percent charged in two minutes. If the method can be commercialized, it could lead to laptops that charge in a few minutes or cell phones that charge in 30 seconds.
Rapid charge and discharge rates have become an important feature of electrical energy storage devices, but cause dramatic reductions in the energy that can be stored or delivered by most rechargeable batteries (their energy capacity). Supercapacitors do not suffer from this problem, but are restricted to much lower stored energy per mass (energy density) than batteries. A storage technology that combines the rate performance of supercapacitors with the energy density of batteries would significantly advance portable and distributed power technology. Here, we demonstrate very large battery charge and discharge rates with minimal capacity loss by using cathodes made from a self-assembled three-dimensional bicontinuous nanoarchitecture consisting of an electrolytically active material sandwiched between rapid ion and electron transport pathways. Rates of up to 400C and 1,000C for lithium-ion and nickel-metal hydride chemistries, respectively, are achieved (where a 1C rate represents a one-hour complete charge or discharge), enabling fabrication of a lithium-ion battery that can be 90% charged in 2 minutes.
The methods used to make the ultrafast-charging electrodes are compatible with a range of battery chemistries; the researchers have also used them to make nickel-metal-hydride batteries, the kind commonly used in hybrid and electric vehicles.
How fast a battery can charge up and then release that power is primarily limited by the movement of electrons and ions into and out of the cathode, the electrode that is negative during recharging. Researchers have been trying to use nanostructured materials to improve the process, but there’s usually a trade-off between total energy storage capacity (which determines how long a battery can run before needing a recharge) and charge rates. “People solved half the problem,” says Paul Braun, professor of materials science and engineering at the University of Illinois at Urbana-Champaign.
Braun’s group has made highly porous metal foams coated with a large amount of active battery materials. The metal provides high electrical conductivity, and even though it’s porous, the structure holds enough active material to store a sufficient amount of energy. The pores allow for ions to move about unimpeded.
9 pages of supplemental information
If you liked this article, please give it a quick review on ycombinator or StumbleUpon. Thanks

Brian Wang is a Futurist Thought Leader and a popular Science blogger with 1 million readers per month. His blog Nextbigfuture.com is ranked #1 Science News Blog. It covers many disruptive technology and trends including Space, Robotics, Artificial Intelligence, Medicine, Anti-aging Biotechnology, and Nanotechnology.
Known for identifying cutting edge technologies, he is currently a Co-Founder of a startup and fundraiser for high potential early-stage companies. He is the Head of Research for Allocations for deep technology investments and an Angel Investor at Space Angels.
A frequent speaker at corporations, he has been a TEDx speaker, a Singularity University speaker and guest at numerous interviews for radio and podcasts. He is open to public speaking and advising engagements.