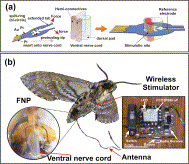
New Scientist – The US Defense Advanced Research Projects Agency (DARPA) has been running a programme to develop machine-insect interfaces for years but electrodes implanted to stimulate the brains or wing muscles of insects were not precise enough. Now Joel Voldman of the Massachusetts Institute of Technology and colleagues have designed a unique, flexible neural probe that can be attached directly to an insect’s ventral nerve cord (VNC), which, along with the brain, makes up the central nervous system in insects.
Another reason previous attempts have not been entirely successful was because the impedance of the electrodes did not match that of the insect’s tissue. This probe is made of a polyimide polymer coated with gold and carbon nanotubes, and its impedance is much closer to that of nerve tissue. One end of the probe is a ring that clamps around the VNC. The inside of the ring has five electrodes which stimulate distinct nerve bundles within the VNC.
Attached to the probe is a wireless stimulator, which contains a radio receiver, as well as a battery and a device to generate electrical pulses. The team implanted the device in the abdomen of a tobacco hawkmoth (Manduca sexta). As it weighs less than half a gram, it is easy for the moth to carry. “Their wingspan is the width of your hand,” says Voldman. “These are big guys.”
i-MAVs and FNP implantation. (a) Schematic of the FNP insertion process, showing how the split-ring can be opened to fit around the VNC. (b) Illustration of i-MAV including moth, FNP and wireless stimulator.
We developed microfabricated flexible neural probes (FNPs) to provide a bi-directional electrical link to the moth Manduca sexta. These FNPs can deliver electrical stimuli to, and capture neural activity from, the insect’s central nervous system. They are comprised of two layers of polyimide with gold sandwiched in between in a split-ring geometry that incorporates the bi-cylindrical anatomical structure of the insect’s ventral nerve cord. The FNPs provide consistent left and right abdominal stimulation both across animals and within an individual animal. The features of the stimulation (direction, threshold charge) are aligned with anatomical features of the moth. We also have used these FNPs to record neuronal activity in the ventral nerve cord of the moth. Finally, by integrating carbon nanotube (CNT)-Au nanocomposites into the FNPs we have reduced the interfacial impedance between the probe and the neural tissue, thus reducing the magnitude of stimulation voltage. This in turn allows use of the FNPs with a wireless stimulator, enabling stimulation and flight biasing of freely flying moths. Together, these FNPs present a potent new platform for manipulating and measuring the neural circuitry of insects, and for other nerves in humans and other animals with similar dimensions as the ventral nerve cord of the moth.
Implantation of FNP into adult moth. (a) Anesthetize moth, ventral side up, box shows incision site; (b) create trap-door incision at base of third abdominal segment; (c) isolate the VNC on glass probe, place FNP nearby; (d) open FNP and bring it onto the VNC, with site 3 penetrating into the connective tissue between the axon-bearing hemiconnectives; (e) position FNP for insertion into the body, VNC is visible passing through split ring; (f) insert FNP so that the VNC lays at its natural depth, align marks with incision edge, add Gelfoam to both sides of FNP; (g) reposition trapdoor piece of cuticle and glue in place; (h) shows actual implanted FNP at conclusion of surgery, animal can be stimulated the next day.
Design of FNPs and anatomical structure of moth’s VNC. (a) Image showing the dimensions of various FNP designs. The standard design uses epoxy connections while the FPC design is matched to flexible printed circuit board connectors. Close-up images of (b) the head showing the location of site 6 reference electrode and holding-tips and (c) the split-ring structure showing the bi-circle design of the FNP. (site areas; 1, 5: 1460 μm2; 2, 4: 900 μm2, 3: 1300 μm2 and 6: 14,400 μm2). (d) Image of a cross section of the VNC of the adult moth overlaid with scaled schematized drawings of the split-ring of the FNP. (e) Histological section of the hemi-connectives showing the distribution of the giant axons.
If you liked this article, please give it a quick review on ycombinator or StumbleUpon. Thanks

Brian Wang is a Futurist Thought Leader and a popular Science blogger with 1 million readers per month. His blog Nextbigfuture.com is ranked #1 Science News Blog. It covers many disruptive technology and trends including Space, Robotics, Artificial Intelligence, Medicine, Anti-aging Biotechnology, and Nanotechnology.
Known for identifying cutting edge technologies, he is currently a Co-Founder of a startup and fundraiser for high potential early-stage companies. He is the Head of Research for Allocations for deep technology investments and an Angel Investor at Space Angels.
A frequent speaker at corporations, he has been a TEDx speaker, a Singularity University speaker and guest at numerous interviews for radio and podcasts. He is open to public speaking and advising engagements.