Researchers at Japan’s Atomic Energy Agency have come up with a new method of processing seawater to extract lithium—an element that plays a key role in advanced batteries for electric vehicles and one that, if current predictions for the EV market prove accurate, could be in short supply before the end of the decade.
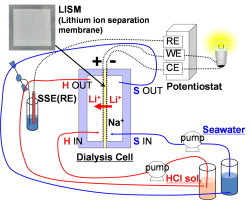
Tsuyoshi Hoshino, a scientist at the JAEA’s Rokkasho Fusion Institute, proposed a method for recovering lithium from seawater using dialysis. Still years from commercialization, the system is based on a dialysis cell with a membrane consisting of a superconductor material. Lithium is the only ion in the seawater that can pass through the membrane, from the negative-electrode side of the cell to the positive-electrode side.
If Hoshino’s method proves efficient and economical, it could transform a market that has seen lots of investment and supposed innovation in recent years but has remained stubbornly resistant to new technologies and new sources of supply. Most lithium is still recovered today in the way it has been for half a century: by evaporating brine collected from salt lakes in enclosed valleys in parts of Chile, Argentina, and Bolivia.
Schematic of Li ion recovery from seawater by electrodialysis by using LISM.
Highlights
• New method for Li recovery from seawater is proposed using the world-first dialysis.
• Li ionic superconductor as the Li separation membrane (LISM) was used.
• Only Li ions can permeate the LISM.
• The Li recovery ratios to approximately 7% after 72 h with no electrical supply.
• Li separation process generates electricity, requiring no external power source.
Abstract
Lithium (Li) procurement is becoming a matter of importance worldwide. Herein, I propose a method for recovering Li from seawater by using world-first dialysis, wherein Li only permeates from the negative electrode side to the positive electrode side through a Li ionic superconductor functioning as a Li separation membrane (LISM). Measurements of the Li ion concentration at the positive electrode side as a function of dialysis duration showed that the Li recovery ratio increased to approximately 7% after 72 h with no applied electric voltage. Moreover, other ions in the seawater did not permeate the LISM. With both ends of the LISM bound with a negative and positive electrode, hydrated Li ion was transformed to Li ion only because Li ion can permeate through the LISM. This new recovery method shows good energy efficiency and is easily scalable and is thus suitable for the industrialised mass production of Li in South American countries, which have briny water containing Li.
Plots of Li recovery ratio on the positive electrode side as a function of the dialysis time.
Only Li ions can significantly permeate this LISM (Lithium Ion Separation Membrane) to pass from the negative electrode side to the positive electrode side. In this Li recovery technique, the negative and positive electrodes are bonded to right and left faces (the two main faces), respectively, of the LISM. Because other ions in seawater (Na, Mg, Ca and K) are not permeated through the LISM, Li becomes selectively concentrated on the positive electrode side. Measurements of the Li ion concentration on the positive electrode side as a function of the dialysis duration with no applied voltage show that the Li recovery ratio increased with time, reaching approximately 7% after 72 h. Furthermore, the Li separation process generates electricity, requiring no external power source. The principal of Li permeation by using LISM is likely similar to that of a concentration cell; thus, the driving force of Li permeation is the differential Li ion concentration between the negative and positive electrode sides.
This world-first recovery method shows good energy efficiency and is easily scalable. It should be suitable for the industrialised mass production of Li from briny water in South America as well as for the recycling of used Li-ion batteries. Furthermore, if other ionic superconductors are developed, this method may also be readily adapted for the recovery of other rare metals from seawater.
SOURCES- MIT Technology Review, Journal Desalination

Brian Wang is a Futurist Thought Leader and a popular Science blogger with 1 million readers per month. His blog Nextbigfuture.com is ranked #1 Science News Blog. It covers many disruptive technology and trends including Space, Robotics, Artificial Intelligence, Medicine, Anti-aging Biotechnology, and Nanotechnology.
Known for identifying cutting edge technologies, he is currently a Co-Founder of a startup and fundraiser for high potential early-stage companies. He is the Head of Research for Allocations for deep technology investments and an Angel Investor at Space Angels.
A frequent speaker at corporations, he has been a TEDx speaker, a Singularity University speaker and guest at numerous interviews for radio and podcasts. He is open to public speaking and advising engagements.