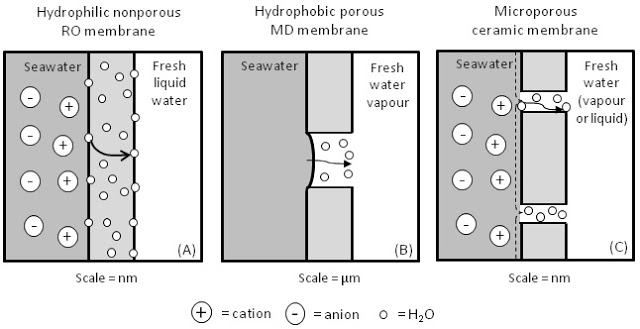
Scientists at Alexandria University in Egypt are developing an innovative water-purifying technique that uses half the energy as previous methods. They have created a membrane that can both clean and desalinate water, that could offer Egypt and other developing countries an inexpensive new water source. The method uses materials from North Africa and could make water desalination a more affordable process, according Digital Trends. Researchers ultimately plan to mass-produce the membrane and print sheets for individual use.
The breakthrough by Alexandria researchers lies in the new kind of membrane with just five “ingredients” that could be made in other labs without great difficulty. It binds with the salt in the water – it even works with the very salty water found in the Red Sea.
“Using pervaporation eliminates the need for electricity that is used in classic desalination processes, thus cutting costs significantly,” Ahmed El-Shafei, an agricultural and biosystems engineering professor at Alexandria University, told Digital Trends.
Abstract – Desalination of simulated seawater by purge-air pervaporation using an innovative fabricated membrane
An innovative polymeric membrane has been invented, which presents a breakthrough in the field of desalination membranes. It can desalinate simulated seawater of exceptionally high concentration to produce a high flux of potable water with over 99.7% salt rejection (%SR) in a once-through purge-air pervaporation (PV) process. A set-up was constructed for conducting the desalination experiments and the effect of initial salt solution concentration (Ci) and pervaporation temperature (Tpv) on the water flux (J), %SR, separation factor, and pervaporation separation index were determined. The membrane was prepared by the phase-inversion technique, of a specially formulated casting solution consisting of five ingredients, after which the membrane was subjected to a post-treatment by which certain properties were conferred. The results confirmed that the salinity of the pervaporate was independent of Ci (all %SR above 99.7). The best result was at Tpv = 70 °C, where J varied from 5.97 to 3.45 l/m2 h for Ci = 40–140 g NaCl/l, respectively. The membrane morphology was confirmed to be asymmetric. The contact angle was immeasurable, indicating the membrane to be super-hydrophilic. Activation energies computed using Arrhenius law were, under all conditions investigated, less than 20 kJ/mol K.

Brian Wang is a Futurist Thought Leader and a popular Science blogger with 1 million readers per month. His blog Nextbigfuture.com is ranked #1 Science News Blog. It covers many disruptive technology and trends including Space, Robotics, Artificial Intelligence, Medicine, Anti-aging Biotechnology, and Nanotechnology.
Known for identifying cutting edge technologies, he is currently a Co-Founder of a startup and fundraiser for high potential early-stage companies. He is the Head of Research for Allocations for deep technology investments and an Angel Investor at Space Angels.
A frequent speaker at corporations, he has been a TEDx speaker, a Singularity University speaker and guest at numerous interviews for radio and podcasts. He is open to public speaking and advising engagements.