Biologists led by Kohji Nishida at Osaka University in Japan have discovered a new way to nurture and grow the many separate tissues that make up the human eyeball, and the scientists need only a small sample adult skin to build them all. Using their new method, Nishida’s team can grow retinas, corneas, the eye’s lens, and more.
In a preliminary trial, the Japanese researchers cultured and grew sheathes of rabbit cornea—the transparent cover of the eye— that restored sight in blind rabbits born without fully-grown corneas.
They could coerce these iPS cells into forming a simple proto-eye, from which they could harvest a bounty of different eye tissues. The researchers kick-start the formation of this proto-eye by growing iPS cells in a petri dish lined with the right combination of proteins and other molecules. Essentially, these proto-eyes amount to four simple rings of different cell types that later transform into different parts of the eye, like the retina or lens. Think of them as one of the earliest and simplest phase in biological eye formation.
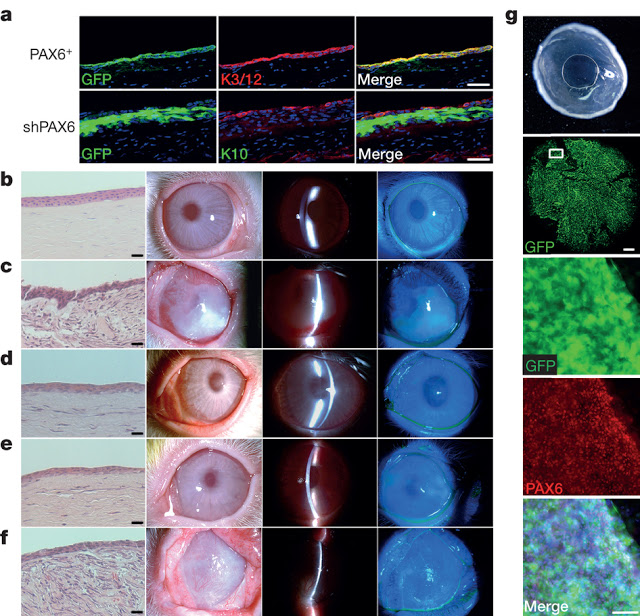
The eye is a complex organ with highly specialized constituent tissues derived from different primordial cell lineages. The retina, for example, develops from neuroectoderm via the optic vesicle, the corneal epithelium is descended from surface ectoderm, while the iris and collagen-rich stroma of the cornea have a neural crest origin. Recent work with pluripotent stem cells in culture has revealed a previously under-appreciated level of intrinsic cellular self-organization, with a focus on the retina and retinal cells. Moreover, we and others have demonstrated the in vitro induction of a corneal epithelial cell phenotype from pluripotent stem cells. These studies, however, have a single, tissue-specific focus and fail to reflect the complexity of whole eye development. Here we demonstrate the generation from human induced pluripotent stem cells of a self-formed ectodermal autonomous multi-zone (SEAM) of ocular cells. In some respects the concentric SEAM mimics whole-eye development because cell location within different zones is indicative of lineage, spanning the ocular surface ectoderm, lens, neuro-retina, and retinal pigment epithelium. It thus represents a promising resource for new and ongoing studies of ocular morphogenesis. The approach also has translational potential and to illustrate this we show that cells isolated from the ocular surface ectodermal zone of the SEAM can be sorted and expanded ex vivo to form a corneal epithelium that recovers function in an experimentally induced animal model of corneal blindness.
SOURCES – Popular Mechanics, Nature

Brian Wang is a Futurist Thought Leader and a popular Science blogger with 1 million readers per month. His blog Nextbigfuture.com is ranked #1 Science News Blog. It covers many disruptive technology and trends including Space, Robotics, Artificial Intelligence, Medicine, Anti-aging Biotechnology, and Nanotechnology.
Known for identifying cutting edge technologies, he is currently a Co-Founder of a startup and fundraiser for high potential early-stage companies. He is the Head of Research for Allocations for deep technology investments and an Angel Investor at Space Angels.
A frequent speaker at corporations, he has been a TEDx speaker, a Singularity University speaker and guest at numerous interviews for radio and podcasts. He is open to public speaking and advising engagements.