Two separate breakthroughs will combine for unlimited youthful blood for antiaging and immune system boosting transfusions
1. Immortalized cell lines can enable factory mass produced red blood cells
2. An activated protein can make blood youthful which boosts immune systems
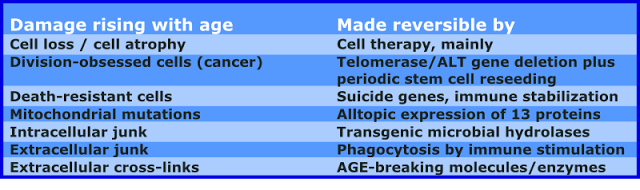
This could be the first cells and tissue that could be immortalized and used for rejuvenation.
It could be possible to have regular resupply (two to four times per year or more often with automation) of youthful cells and tissues for health maintenance and as antiaging treatment. This would be combined with treatments to clear old cells more rapidly from the body.
Researchers have generated the first immortalised cell lines which allow more efficient manufacture of red blood cells. The team, from the University of Bristol and NHS Blood and Transplant, were able to manufacture red blood cells in a more efficient scale than was previously possible.
The results, published in Nature Communications, could, if successfully tested in clinical trials, eventually lead to a safe source of transfusions for people with rare blood types, and in areas of the world where blood supplies are inadequate or unsafe.
Previously, research in this field focused on growing donated stem cells straight into mature red blood cells. However that method presently produces small numbers of mature cells and requires repeat donations.
The world-leading team in Bristol have now developed a robust and reproducible technique which allows the production of immortalised erythroid cell lines from adult stem cells. These premature red cells can be cultured indefinitely, allowing larger-scale production, before being differentiated into mature red blood cells.
Dr Jan Frayne, from the University of Bristol’s School of Biochemistry, said: “Previous approaches to producing red blood cells have relied on various sources of stem cells which can only presently produce very limited quantities. By taking an alternative approach we have generated the first human immortalised adult erythroid line (Bristol Erythroid Line Adult or BEL-A), and in doing so, have demonstrated a feasible way to sustainably manufacture red cells for clinical use from in vitro culture.
NHS Blood and Transplant needs to collect 1.5 million units of blood each year to meet the needs of patients across England and the ongoing need for life saving blood donations remains. It would be many years before manufactured cells could be available on a large scale.
NHS Blood and Transplant announced plans for in-man trials of manufactured blood in 2015. This first trial will not use Bel-A cells. The first trial, due to start by the end of 2017, will use manufactured red cells from stem cells in a normal blood donation.
Continuously expanding cells – images of the immortalised early erythroid cells, labelled with number of days since immortalisation, demonstrating no change in morphology of the cells with extended time in continuous culture.
Separate work makes blood young which boosts immune systems
Researchers examined the bone marrow in mice, they found that older animals have much lower levels of a protein called osteopontin. To see if this protein has an effect on blood stem cells, the team injected stem cells into mice that lacked osteopontin and found that the cells rapidly aged.
But when older stem cells were mixed in a dish with osteopontin and a protein that activates it, they began to produce white blood cells just as young stem cells do. This suggests osteopontin makes stem cells behave more youthfully. “If we can translate this into a treatment, we can make old blood young again,” Geiger says.
Geiger’s team is developing a drug containing osteopontin and the activating protein to encourage blood stem cells to behave more youthfully. “It should boost the immune system of elderly people,” he says.
Such a drug might have benefits beyond fighting infection and alleviating anaemia. The team also think the protein will boost levels of mother stem cells. Having only a small number of such cells has been linked to heart disease, so Geiger says there is a chance that boosting them may help prevent this.
Osteopontin might also be useful for treating age-linked blood disorders, such as myelodysplasias that involve dysfunctional cells, says Martin Pera of the Jackson Laboratory in Bar Harbor, Maine. “It is possible that rejuvenating bone marrow stem cells could help with these conditions,” he says.
Osteopontin attenuates aging‐associated phenotypes of hematopoietic stem cells
Aging of bone marrow hematopoietic stem cells (HSCs) is thought to arise primarily from stem cell intrinsic mechanisms. Here, reduced levels of the secreted matrix glycoprotein osteopontin (OPN) in aged stroma are found to cause aging‐associated features in HSCs.
- Transplantation of young bone marrow cells into an aged microenvironment increases HSC frequency and skews myeloid development.
- Aging alters the cellular composition of the endosteal stroma, decreasing osteoblasts.
- Secreted levels of stromal OPN are diminished in the stem cell niche upon aging.
- Transplantation of young HSCs into OPN knockout decreases engraftment and results in HSC expansion, loss of stem cell polarity and augmented Cdc42 activity, thus mimicking old stroma.
- Treating aged HSCs with a thrombin‐cleaved OPN fragment leads to phenotypical and functional rejuvenation by activating integrin α9β1 on HSCs.
Abstract
Upon aging, hematopoietic stem cells (HSCs) undergo changes in function and structure, including skewing to myeloid lineages, lower reconstitution potential and loss of protein polarity. While stem cell intrinsic mechanisms are known to contribute to HSC aging, little is known on whether age‐related changes in the bone marrow niche regulate HSC aging. Upon aging, the expression of osteopontin (OPN) in the murine bone marrow stroma is reduced. Exposure of young HSCs to an OPN knockout niche results in a decrease in engraftment, an increase in long‐term HSC frequency and loss of stem cell polarity. Exposure of aged HSCs to thrombin‐cleaved OPN attenuates aging of old HSCs, resulting in increased engraftment, decreased HSC frequency, increased stem cell polarity and a restored balance of lymphoid and myeloid cells in peripheral blood. Thus, our data suggest a critical role for reduced stroma‐derived OPN for HSC aging and identify thrombin‐cleaved OPN as a novel niche informed therapeutic approach for ameliorating HSC phenotypes associated with aging.

Brian Wang is a Futurist Thought Leader and a popular Science blogger with 1 million readers per month. His blog Nextbigfuture.com is ranked #1 Science News Blog. It covers many disruptive technology and trends including Space, Robotics, Artificial Intelligence, Medicine, Anti-aging Biotechnology, and Nanotechnology.
Known for identifying cutting edge technologies, he is currently a Co-Founder of a startup and fundraiser for high potential early-stage companies. He is the Head of Research for Allocations for deep technology investments and an Angel Investor at Space Angels.
A frequent speaker at corporations, he has been a TEDx speaker, a Singularity University speaker and guest at numerous interviews for radio and podcasts. He is open to public speaking and advising engagements.