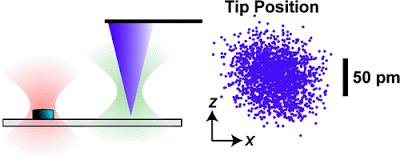
Instrumental drift in atomic force microscopy (AFM) remains a critical, largely unaddressed issue that limits tip−sample stability, registration, and the signal-to-noise ratio during imaging. By scattering a laser off the apex of a commercial AFM tip, we locally measured and thereby actively controlled its three-dimensional position above a sample surface to
In an atomic force microscope (AFM), force is measured by a laser beam (yellow in this artist’s rendition) bouncing off the diving-board like cantilever. To make an ultrastable AFM, researchers at JILA added two other lasers (green and red) to measure the three dimensional position of both the tip and a reference mark in the sample. These measurements allow researchers to remove drift and vibration in the instrument’s measurements caused by environmental factors.FURTHER READING
The JILA research group at the University of Colorado.While extremely sensitive to atomic-scale features, AFMs also are extremely sensitive to interference from acoustic noise, temperature shifts and vibration, among other factors. This makes it difficult or impossible either to hold the probe in one place to observe the specimen under it over time (useful for studying the dynamics of proteins) or to move the probe away and return to exactly the same spot (potentially useful for nanoscale manufacturing). “At this scale, it’s like trying to hold a pen and draw on a sheet of paper while riding in a jeep,” observes NIST physicist Thomas Perkins. A few instruments in specialized labs, including some at NIST, solve this problem by operating at extremely cold temperatures in ultra-high vacuums and in heavily isolated environments, but those options aren’t available for the vast majority of AFMs, particularly those used in bioscience laboratories where the specimen often must be immersed in a fluid.
The JILA solution uses two additional laser beams to sense the three-dimensional motion of both the test specimen and the AFM probe. The beams are held stable relative to each other to provide a common reference. To hold the specimen, the team uses a transparent substrate with tiny silicon disks—“fiducial marks”—embedded in it at regular intervals. One laser beam is focused on one of these disks. A small portion of the light scatters backwards to a detector. Any lateral vibration or drift of the sample shows up at the detector as a motion of the spot while any vertical movement shows up as a change in light intensity.** A similar trick with the second beam is used to detect vibration or drift in the probe tip, with the added complication that the system has to work with the scant amount of light reflected off the apex of the AFM probe. Unwanted motion of the tip relative to the sample is corrected on the fly by moving the substrate in the opposite direction. “This is the same idea as active noise cancellation headphones, but applied to atomic force microscopy,” says Perkins.
Brian Wang is a Futurist Thought Leader and a popular Science blogger with 1 million readers per month. His blog Nextbigfuture.com is ranked #1 Science News Blog. It covers many disruptive technology and trends including Space, Robotics, Artificial Intelligence, Medicine, Anti-aging Biotechnology, and Nanotechnology.
Known for identifying cutting edge technologies, he is currently a Co-Founder of a startup and fundraiser for high potential early-stage companies. He is the Head of Research for Allocations for deep technology investments and an Angel Investor at Space Angels.
A frequent speaker at corporations, he has been a TEDx speaker, a Singularity University speaker and guest at numerous interviews for radio and podcasts. He is open to public speaking and advising engagements.