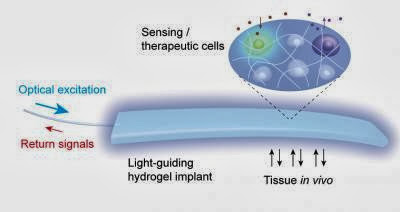
Light can now be used to heal diabetes in mice. By implanting a transparent gel that contains genetically modified light-sensitive cells, researchers have demonstrated a new type of implant that could one day be used to treat disease and monitor toxins in people.
“Light is a great tool to interface with biological systems, but there is a fundamental problem. It gets scattered when it hits tissue, and at depths much thinner than our skin,” says lead author Myunghwan Choi of Harvard Medical School in Boston.
Choi and his colleagues designed an implantable gel that could get around this, by guiding light under the mouse’s skin. In experiments, the team impregnated the gel with different types of genetically modified cells before implanting it.
To control diabetes, the team shone light into the mouse and at the implanted gel using a fibre optic cable attached to its head. The light triggered cells in the gel to produce a compound that stimulated the secretion of insulin and stabilised blood glucose levels. Separately, the team also showed they could monitor for cadmium poisoning using cells that fluoresced when the mouse was under stress from the toxin.
Nature Photonics – Light-guiding hydrogels for cell-based sensing and optogenetic synthesis in vivo
The use of light to communicate with cells has been restricted by its limited ability to pass through tissues. Now researchers at the Wellman Center for Photomedicine at Massachusetts General Hospital have developed a way to deliver a light signal to specific tissues deep within the body.
Light passing through an optical fiber (left) can either carry in a signal that stimulates the activity of cells embedded in the hydrogel implant or bring back a signal generated by cells responding to something in their environment. Credit: (Harvard Bio-Optics Lab/Wellman Center for Photomedicine, Mass. General Hospital)
“One of the best known example is use of optogenetics – activation or deactivation of brain cells by illumination with different colors of light – to treat brain disorders. But how to deliver light deep within the brain or other tissues has been a common problem. The implant we have developed may help solve this problem.”
Called a light-guiding hydrogel, the implant is constructed from a polymer-based scaffolding capable of supporting living cells and contains cells genetically engineered either to carry out a specific activity in response to light or to emit light in response to a particular metabolic signal. An optical fiber connects the implant to either an external light source or a light detector.
The investigators first determined the properties of the hydrogel scaffolding – including transparency, flexibility and stability – that would be most appropriate for delivering or detecting a light signal. After determining how many cells could be implanted into the hydrogel without significantly reducing its ability to transmit a light signal, they developed and tested in mice two different systems, both involving implantation of a 4-centimeter hydrogel beneath the animal’s skin.
The first system’s implants contained cells genetically engineered to express light-emitting green fluorescent protein (GFP) upon contact with a toxin. After confirming in vitro the hydrogels’ response to nanoparticles containing the toxic metal cadmium, the researchers implanted the hydrogels beneath the skin of three groups of mice. One group was then injected with the cadmium nanoparticles, the second received nanoparticles encased in a polymer shell that shielded cells from the toxin, and the third received a control saline injection. The implants only produced a GFP-signal in response to the unshielded nanoparticles, indicating their ability to sense a change – in this instance the presence of a toxin – in the cellular environment.
To investigate a possible therapeutic application for the system, the investigators used a hydrogel implant containing cells that respond to blue light by producing glucagon-like peptide-1 (GLP-1), a protein playing an essential role in glucose metabolism. After the implants were placed under the skin of mice with diabetes, the blue light signal was delivered for 12 hours. A day and a half later – 48 hours after the implant – the animals that received the light signal had double the level of GLP-1 in their blood, along with significantly better results in a glucose tolerance test, than did implanted mice not treated with light.
“This work combines several existing technologies well known in their respective fields – such as drug delivery, genetic engineering, biomaterial science, and photonics – to build a new implant system that enables the delivery of photomedicine deep in the body,” says Yun, an associate professor of Dermatology at Harvard Medical School and director of the Harvard Bio-Optics Lab. “This is the first time anyone has shown the ability to talk optically – by means of light – with cells deep within the body, both to sense the presence of a toxin and to deliver a cell-based therapy.”
The researchers add that future studies should investigate how changing the shape and structure of the hydrogel can improve the implant’s light-guiding properties, ways to improve the production and delivery of a therapeutic protein, how the immune system would react to long-term implantation and ways to deliver or detect the light signal that would not require passing a fiber through the skin.
SOURCE – Eurekalert, New Scientist
If you liked this article, please give it a quick review on ycombinator or StumbleUpon. Thanks

Brian Wang is a Futurist Thought Leader and a popular Science blogger with 1 million readers per month. His blog Nextbigfuture.com is ranked #1 Science News Blog. It covers many disruptive technology and trends including Space, Robotics, Artificial Intelligence, Medicine, Anti-aging Biotechnology, and Nanotechnology.
Known for identifying cutting edge technologies, he is currently a Co-Founder of a startup and fundraiser for high potential early-stage companies. He is the Head of Research for Allocations for deep technology investments and an Angel Investor at Space Angels.
A frequent speaker at corporations, he has been a TEDx speaker, a Singularity University speaker and guest at numerous interviews for radio and podcasts. He is open to public speaking and advising engagements.