AScientists at The Scripps Research Institute (TSRI) have found a way to change leukemia cells into leukemia-killing immune cells. The surprise finding could lead to a powerful new therapy for leukemia and possibly other cancers.
“It’s a totally new approach to cancer, and we’re working to test it in human patients as soon as possible,” said senior investigator Richard A. Lerner, Institute Professor and the Lita Annenberg Hazen Professor of Immunochemistry at TSRI.
The findings, published this week in the Proceedings of the National Academy of Sciences, result from the discovery of a rare human antibody.
Unexpected Effects
The Lerner laboratory has pioneered techniques to generate and screen very large libraries of antibodies (immune system molecules), using the power of large numbers to find therapeutic antibodies that bind to a desired target or activate a desired receptor on cells.
Recently, the lab mounted an effort to find therapies for people with certain immune cell or blood factor deficiencies, by looking for antibodies that activate growth-factor receptors on immature bone marrow cells that might induce these bone marrow cells to mature into specific blood cell types. Over the past few years, Lerner and his team succeeded in identifying a number of antibodies that activate marrow-cell receptors in this way.
Transformation
In the new study, Richard A. Lerner, institute professor and the Lita Annenberg Hazen professor of Immunochemistry at TSRI and senior investigator, teamed up with colleagues, including first author Kyungmoo Yea, an assistant professor of cellular and molecular biology at TSRI. They decided to test 20 of the recently discovered receptor-activating antibodies on acute myeloid leukemia cells taken from human patients. One of the antibodies ended up having an incredible impact on the leukemia cells.
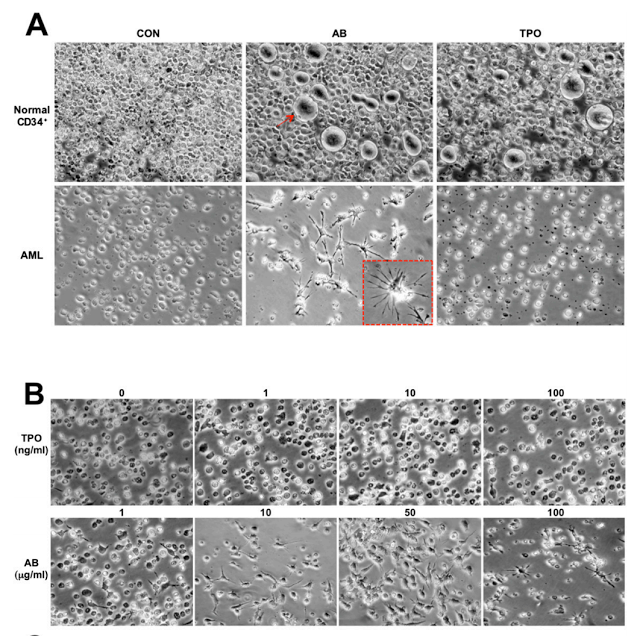
(A) Normal BM CD34+ cells and AML cells after 4 d culture in the presence of PBS, antibody (10 μg/mL), or TPO (10 ng/mL). The red arrow indicates a megakaryocyte. The red-boxed Inset shows an enlarged image of a differentiated cell. (B) The AML cells after 4 d culture with various concentrations of antibody
PNAS – Agonist antibody that induces human malignant cells to kill one another
Significance
A major goal in cancer research is to discover agents that transform malignant cells into benign cells. Here, we report on an agonist antibody that converts leukemic cells into killer cells. This induction has an added benefit: In addition to transforming the cancer cells into other, presumably less aggressive, cells, the newly induced cells have a killer phenotype and kill other as yet unconverted members of the malignant clone.
Abstract
An attractive, but as yet generally unrealized, approach to cancer therapy concerns discovering agents that change the state of differentiation of the cancer cells. Recently, we discovered a phenomenon that we call “receptor pleiotropism” in which agonist antibodies against known receptors induce cell fates that are very different from those induced by the natural agonist to the same receptor. Here, we show that one can take advantage of this phenomenon to convert acute myeloblastic leukemic cells into natural killer cells. Upon induction with the antibody, these leukemic cells enter into a differentiation cascade in which as many as 80% of the starting leukemic cells can be differentiated. The antibody-induced killer cells make large amounts of perforin, IFN-γ, and granzyme B and attack and kill other members of the leukemic cell population. Importantly, induction of killer cells is confined to transformed cells, in that normal bone marrow cells are not induced to form killer cells. Thus, it seems possible to use agonist antibodies to change the differentiation state of cancer cells into those that attack and kill other members of the malignant clone from which they originate.
More background
A high percentage of acute myeloid leukemia cells express the thrombopoietin (TPO) receptor, and the effective antibody was a highly potent and selective activator of this receptor on marrow cells. When the antibody was applied to healthy immature marrow cells, it caused them to mature into blood-platelet-producing cells called megakaryocytes. However, when the antibody was applied to acute myeloid leukemia cells, they matured into very different cells known as dendritic cells, key support cells in the immune system.
By itself, this could be a valuable therapeutic strategy, but it wasn’t the end of the story. Lerner’s team noted that, with longer exposures to the antibodies and certain other lab-dish conditions, the induced dendritic cells developed further–into cells that closely resembled natural killer (NK) cells.
NK cells represent one of the rapid-reaction forces of the immune system. They can be effective against viruses and bacteria–and cancer cells–even without prior exposure. They don’t have highly specific receptors for recognizing individual targets, as T-cells do, but instead are capable of detecting, in a general way, when a nearby cell is infected or cancerous.
“That antibody could have turned those acute myeloid leukemia cells into a lot of other cell types, but somehow we were lucky enough to get NK cells,” Lerner said.
‘Fratricide’
The team examined these induced NK cells with electron microscopy and observed that many of the cells had extended tendrils through the outer membranes of neighboring leukemic cells–their erstwhile brethren. In lab dish tests, a modest number of these NK cells wiped out about 15 percent of the surrounding acute myeloid leukemia cell population in just 24 hours.
Curiously, the induced NK cells’ cancer-killing effect appeared to be purely fratricidal. The researchers found that unrelated breast cancer cells did not die off in large numbers when in the presence of the NK cells.
Why the induced NK cells appear to target only closely related cells isn’t yet clear. In principle, though, there are yet-to-be-discovered antibodies–and even small-molecule compounds–that would turn other cancerous cell types into fratricidal NK cells, by activating other receptors expressed on those cells.
Such fratricidal therapies, which Lerner terms “fratricidins,” would have several potential advantages. First, especially if they are antibodies, they could be clinically useful with little or no further modification. Second, their high specificity for their target receptors, and the resulting NK cells’ specificity for related cancer cells, should reduce the likelihood of adverse side effects, possibly making them much more tolerable than traditional cancer chemotherapies.
Finally, the peculiar dynamics of fratricidin therapy, in which every cancerous cell is potentially convertible to a cancer-killing NK cell, suggests that–if the strategy works–it might not just reduce the targeted cancer-cell population in a patient, but eliminate it altogether.
“We’re in discussions with pharmaceutical companies to take this straight into humans after the appropriate preclinical toxicity studies,” he said.
SOURCES – Eurekalert, PNAS

Brian Wang is a Futurist Thought Leader and a popular Science blogger with 1 million readers per month. His blog Nextbigfuture.com is ranked #1 Science News Blog. It covers many disruptive technology and trends including Space, Robotics, Artificial Intelligence, Medicine, Anti-aging Biotechnology, and Nanotechnology.
Known for identifying cutting edge technologies, he is currently a Co-Founder of a startup and fundraiser for high potential early-stage companies. He is the Head of Research for Allocations for deep technology investments and an Angel Investor at Space Angels.
A frequent speaker at corporations, he has been a TEDx speaker, a Singularity University speaker and guest at numerous interviews for radio and podcasts. He is open to public speaking and advising engagements.