In The Journal of Physical Chemistry, Assistant Professor of Chemical Engineering Jose L. Mendoza-Cortes details how this new material efficiently captures sunlight and then, how the energy can be used to break down water into oxygen (O2) and hydrogen (H2). This process is known as oxidation, and it is also what happens during photosynthesis when a plant uses light to break down water and carbohydrates, which are the main energy sources for the plant.
His discovery generates exciting new prospects for how this process could be used to forge new energy sources in a carbon neutral way. Potentially, hydrogen could be transported to other locations and burned as fuel.
“In theory, this should be a self-sustaining energy source,” Mendoza-Cortes said. “Perhaps in the future, you could put this material on your roof and it could turn rain water into energy with the help of the sun.”
But, unlike many other energy sources, this won’t have a negative effect on the environment.
“You won’t generate carbon dioxide or waste,” he said.
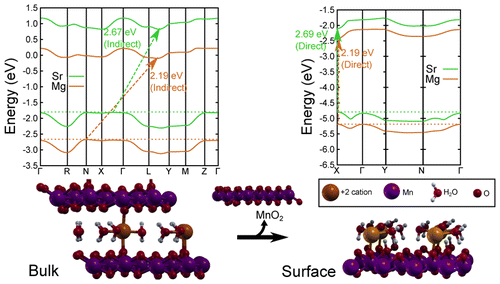
Mendoza-Cortes, a computational and theoretical chemist, said the challenge he faced was designing something that didn’t rust from the process of breaking down water that also trapped the energy and was inexpensive to create. To do this, he initially developed a multilayered material out of manganese oxide, commonly known as birnessite.
But something exciting happened when Mendoza-Cortes and his team peeled back the layers of the material so just a single layer of the material remained — it began trapping light at a much faster rate.
In technical terms, it transitioned from an indirect band gap material to a direct band gap one.
Light with photo energy can penetrate indirect band gap materials much more easily without getting absorbed and used for other purposes. Silicon, for example, is the most commonly known indirect gap band material. But to make the material effective, silicon solar cells are typically stacked and thus hundreds of micrometers thick. If they were any thinner, light would simply pass through them.
Creating a single-layer material that can efficiently trap light is a much more desirable outcome because it is much simpler and cheaper to manufacture.
“This is why the discovery of this direct band gap material is so exciting,” Mendoza-Cortes said. “It is cheap, it is efficient and you do not need a large amount to capture enough sunlight to carry out fuel generation.”
Mendoza-Cortes came to FSU by way of the Energy and Materials Strategic Faculty Hiring Initiative. He is a researcher at FSU’s High-Performance Materials Institute (HPMI), a multidisciplinary research institute dedicated to the research and development of advanced materials and manufacturing technologies.
Abstract
We show a comprehensive study on the structure and electronic properties of a layered manganese oxide commonly known as birnessite. We present the effects of substituting different intercalated cations (Li+, Na+, K+, Be2+, Mg2+, Ca2+, Sr2+, Zn2+, B3+, Al3+, Ga3+, Sc3+, and Y3+) and the role of waters in the intercalated layer. The importance of the Jahn–Teller effect and ordering of the Mn3+ centers due to cation intercalation are addressed to explain the ability to tune the indirect band gap (Egi) from 2.63 to ∼2.20 eV and the direct band gap (Egd) from 3.09 to ∼2.50 eV. By aligning the structures’ bands, we noted that structures with Sr, Ca, B, and Al have potential for usage in water splitting, and anhydrous B-birnessite is predicted to have a suitable direct band gap for light capturing. Furthermore, we also demonstrate how the effects of cations in the bulk differ from the behavior on single layer surfaces. More specifically, we show that an indirect to direct band transition is observed when we separate the bulk into a single layer oxide. This study shows a new strategy for tuning the band gap of layered materials to capture light which may couple to its intrinsic water-splitting catalytic properties, thus resembling photosynthesis.
SOURCES – Florida State University, Journal of Physical Chemistry

Brian Wang is a Futurist Thought Leader and a popular Science blogger with 1 million readers per month. His blog Nextbigfuture.com is ranked #1 Science News Blog. It covers many disruptive technology and trends including Space, Robotics, Artificial Intelligence, Medicine, Anti-aging Biotechnology, and Nanotechnology.
Known for identifying cutting edge technologies, he is currently a Co-Founder of a startup and fundraiser for high potential early-stage companies. He is the Head of Research for Allocations for deep technology investments and an Angel Investor at Space Angels.
A frequent speaker at corporations, he has been a TEDx speaker, a Singularity University speaker and guest at numerous interviews for radio and podcasts. He is open to public speaking and advising engagements.