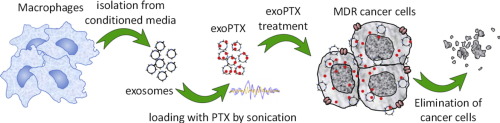
The cancer drug paclitaxel just got more effective. For the first time, researchers from the University of North Carolina at Chapel Hill have packaged it in containers derived from a patient’s own immune system, protecting the drug from being destroyed by the body’s own defenses and bringing the entire payload to the tumor.
“That means we can use 50 times less of the drug and still get the same results,” said Elena Batrakova, Ph.D., an associate professor in the UNC Eshelman School of Pharmacy. “That matters because we may eventually be able to treat patients with smaller and more accurate doses of powerful chemotherapy drugs resulting in more effective treatment with fewer and milder side effects.”
The work, led by Batrakova and her colleagues at the UNC Eshelman School of Pharmacy’s Center for Nanotechnology in Drug Delivery, is based on exosomes, which are tiny spheres harvested from the white blood cells that protect the body against infection. The exosomes are made of the same material as cell membranes, and the patient’s body doesn’t recognize them as foreign, which has been one of the toughest issues to overcome in the past decade with using plastics-based nanoparticles as drug-delivery systems.
“Exosomes are engineered by nature to be the perfect delivery vehicles,” said Batrakova, who has also used this technique as a potential therapy for Parkinson’s disease. “By using exosomes from white blood cells, we wrap the medicine in an invisibility cloak that hides it from the immune system. We don’t know exactly how they do it, but the exosomes swarm the cancer cells, completely bypassing any drug resistance they may have and delivering their payload.”
Paclitaxel is a potent drug used in the United States as a first- and second-line treatment for breast, lung and pancreatic cancers. It can have serious and unpleasant side effects, such as hair loss, muscle and joint pain and diarrhea, and it can put patients at greater risk of serious infection.
In their experiment, Batrakova’s team extracted exosomes from mouse white blood cells and loaded them with paclitaxel. They then tested the treatment — which they call exoPXT — against multiple-drug-resistant cancer cells in petri dishes. The team saw that they needed 50 times less exoPXT to achieve the same cancer-killing effect as formulations of the drug currently being used, such as Taxol.
The researchers next tested the therapy in mouse models of drug-resistant lung cancer. They loaded the exosomes with a dye in order to track their progress through the lungs and found that the exosomes were thorough in seeking out and marking cancer cells, making them a surprisingly effective diagnostic tool in addition to being a powerful therapeutic.
“Accurately mapping the extent of tumors in the lungs is one of the biggest challenges in treating lung-cancer patients,” said Batrakova. “Our results show how powerful exosomes can be as both a therapeutic and a diagnostic.”
Abstract
Exosomes have recently come into focus as “natural nanoparticles” for use as drug delivery vehicles. Our objective was to assess the feasibility of an exosome-based drug delivery platform for a potent chemotherapeutic agent, paclitaxel (PTX), to treat MDR cancer. Herein, we developed and compared different methods of loading exosomes released by macrophages with PTX (exoPTX), and characterized their size, stability, drug release, and in vitro antitumor efficacy. Reformation of the exosomal membrane upon sonication resulted in high loading efficiency and sustained drug release. Importantly, incorporation of PTX into exosomes increased cytotoxicity more than 50 times in drug resistant MDCKMDR1 (Pgp+) cells. Next, our studies demonstrated a nearly complete co-localization of airway-delivered exosomes with cancer cells in a model of murine Lewis lung carcinoma pulmonary metastases, and a potent anticancer effect in this mouse model. We conclude that exoPTX holds significant potential for the delivery of various chemotherapeutics to treat drug resistant cancers.
SOURCES – University of North Carolina, Nanomedicine: Nanotechnology, Biology and Medicine.

Brian Wang is a Futurist Thought Leader and a popular Science blogger with 1 million readers per month. His blog Nextbigfuture.com is ranked #1 Science News Blog. It covers many disruptive technology and trends including Space, Robotics, Artificial Intelligence, Medicine, Anti-aging Biotechnology, and Nanotechnology.
Known for identifying cutting edge technologies, he is currently a Co-Founder of a startup and fundraiser for high potential early-stage companies. He is the Head of Research for Allocations for deep technology investments and an Angel Investor at Space Angels.
A frequent speaker at corporations, he has been a TEDx speaker, a Singularity University speaker and guest at numerous interviews for radio and podcasts. He is open to public speaking and advising engagements.