WHO Director-General, Margaret Chan, will convene an International Health Regulations Emergency Committee on Zika virus and observed increase in neurological disorders and neonatal malformations
Outbreak in the Americas
In May 2015, Brazil reported its first case of Zika virus disease. Since then, the disease has spread within Brazil and to 22 other countries and territories in the region.
Arrival of the virus in some countries of the Americas, notably Brazil, has been associated with a steep increase in the birth of babies with abnormally small heads and in cases of Guillain-Barré syndrome, a poorly understood condition in which the immune system attacks the nervous system, sometimes resulting in paralysis.
A causal relationship between Zika virus infection and birth defects and neurological syndromes has not been established, but is strongly suspected.
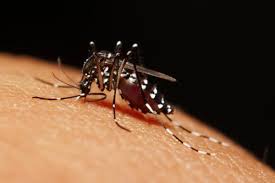
- Zika virus disease is caused by a virus transmitted by Aedes mosquitoes.
- People with Zika virus disease usually have a mild fever, skin rash (exanthema) and conjunctivitis. These symptoms normally last for 2-7 days.
- There is no specific treatment or vaccine currently available.
- The best form of prevention is protection against mosquito bites.
- The virus is known to circulate in Africa, the Americas, Asia and the Pacifi
Zika virus is transmitted to people through the bite of an infected mosquito from the Aedes genus, mainly Aedes aegypti in tropical regions. This is the same mosquito that transmits dengue, chikungunya and yellow fever.
The Zika virus, linked to severe birth defects in thousands of babies in Brazil, is “spreading explosively” and could infect as many as 4 million people in the Americas, the World Health Organization (WHO) said on Thursday.
There is no vaccine or treatment for Zika, which is like dengue and causes mild fever, rash and red eyes. An estimated 80 percent of people infected have no symptoms. Much of the effort against the illness focuses on protecting people from mosquitoes and reducing mosquito populations.
Developing a safe and effective vaccine could take a year, WHO Assistant Director Bruce Aylward said, and it would take six to nine months just to confirm whether Zika is the actual cause of the birth defects, or if the two are just associated.
Gene drive could be used to eliminate the Aedes mosquitos
The idea sounds appealingly simple: Quickly spread a gene through a population of animals in order to prevent it from transmitting disease, or, more directly, to kill a destructive species such as an agricultural pest. Gene-drive technology is at the heart of such concepts. Gene drive shifts biases inheritance to favor certain versions of genes, a genetic alteration introduced into a few members of a population spreads rapidly throughout the entire population. If that alteration inhibits reproduction or survival in some way, gene drive can drive that population extinct in theory. In other uses, a desired trait could be driven through a population.
In July, geneticists showed that one gene drive system was almost 100% effective in spreading a mutated pigmentation gene through a population of lab fruit flies, fueling fears about the power of gene drive.
There are a number of reasons why gene drives may not be as useful, or scary, as some think:
- Gene drive works only in sexually reproducing species, and the genetic change spreads further with each successive generation. So changing or eliminating a population is practical only if the species has a short generation time—like Drosophila, or mosquitos. With many vertebrates, it would take decades for an introduced gene mutation or trait to spread wide enough to make a difference.
- It has not yet been demonstrated that a CRISPR-Cas9 gene-driven change persists across many generations. The paper where gene drive was so effective only reported on one generation. For mosquitos, in which researchers want to knock out populations near people or introduce a parasite-resistant gene, modeling efforts indicate that drive would have to persist 20 generations to spread completely, Burt says.
- There are few, if any, organisms so well characterized, say biologists, that they can predict the ecological effect of a gene-driven change or a disappearing population. We will “have the ability not just to change the genome but [also] to change the balance of species in a community,” says Allison Snow, a plant biologist from Ohio State University, Columbus. “There’s a lot of potential for ignorance, human error, or intent to cause harm.”
- Before gene drive can be applied to wild populations instead of well-characterized laboratory ones, the CRISPR-Cas9 genome–editing technology needs to become even more precise. As Shengdar Tsai, a CRISPR researcher at Massachusetts General Hospital in Boston, pointed out, his team’s analysis of the method in human cells uncovered about two dozen so-called off-target effects—places where the DNA not meant to be changed was. The sites identified confirm that the sites affected by CRISPR-Cas9 can be difficult to predict.
- Instead of using gene drive to make malaria-carrying mosquitos extinct, a less ecologically worrisome strategy would be to change the insect’s genome so it would not transmit the malaria parasite to humans. But researchers don’t know enough about the mosquito immune system to target a specific gene for this type of gene drive yet, Burt says.
- An effective “fail-safe” strategy that would cause gene drive to peter out after a specified number of generations or because researchers decide they needed to stop a gene’s spread still needs to be developed. One of the more promising ones—to undo the genetic change with another gene drive effort—may still be problematic if it’s gene drive itself that goes awry.
- Hybridization between closely related animal species needs to be better understood before gene drives are unleashed. Successful mating between two species results in so-called gene flow, which could allow a gene-driven mutation to hop into an unintended species. This could be useful for malaria control—a gene drive given to one parasite-carrying mosquito species could spread it to the other seven that carry the human pathogen. But in other scenarios, a species-hopping gene drive could lead to the demise of the wrong species.
ormally, it takes years for genetic changes to spread through a population. That is because, during sexual reproduction, each of the two versions of any gene has only a fifty per cent chance of being inherited. But a gene drive which is named for its ability to propel genes through populations over many generations manages to override the traditional rules of genetics. A mutation made by CRISPR on one chromosome can copy itself in every generation, so that nearly all descendants would inherit the change. A mutation engineered into a mosquito that would block the parasite responsible for malaria, for instance, could be driven through a large population of mosquitoes within a year or two. If the mutation reduced the number of eggs produced by that mosquito, the population could be wiped out, along with any malaria parasites it carried.
Kevin Esvelt, an evolutionary biologist at Harvard, was the first to demonstrate how gene drives and CRISPR could combine to alter the traits of wild populations. Recently, he has begun to study the possibility of using the technology to eliminate Lyme disease by rewriting the genes of mice in the wild. Lyme disease is caused by a bacterium and transmitted by ticks, and more than eighty-five per cent of the time they become infected after biting a mouse. Once exposed, however, some mice naturally acquire resistance or immunity.
SOURCE – Journal Science by Elizabeth Pennisi, World Health Organization, Reuters, Science

Brian Wang is a Futurist Thought Leader and a popular Science blogger with 1 million readers per month. His blog Nextbigfuture.com is ranked #1 Science News Blog. It covers many disruptive technology and trends including Space, Robotics, Artificial Intelligence, Medicine, Anti-aging Biotechnology, and Nanotechnology.
Known for identifying cutting edge technologies, he is currently a Co-Founder of a startup and fundraiser for high potential early-stage companies. He is the Head of Research for Allocations for deep technology investments and an Angel Investor at Space Angels.
A frequent speaker at corporations, he has been a TEDx speaker, a Singularity University speaker and guest at numerous interviews for radio and podcasts. He is open to public speaking and advising engagements.