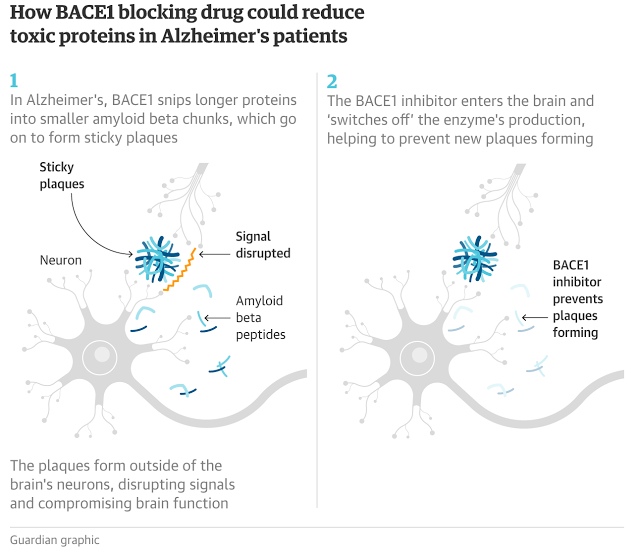
If the tablet, produced by pharmaceutical giant Merck, is also shown to slow the pace of mental decline – a crucial question that a major clinical trial should answer when it reports next year – it could be the first treatment for Alzheimer’s to be licensed in more than a decade.
32 patients with early stage Alzheimer’s disease were given the drug, called verubecestat, daily for seven days. Healthy volunteers were also given the drug for up to two weeks.
This was not long enough to show visible changes to the accumulation of plaques in the brain, by MRI scans for instance. However, samples taken from the fluid surrounding the brain showed the drug had reduced the levels of two compounds that are known to be the building blocks for abnormal amyloid proteins.
Hardy said that the changes to the biomarkers convince him that the drug is successfully targeting the buildup of plaques in the brain. The real remaining uncertainty, he said, was whether this would convert into cognitive benefits for patients.
Getting to first BACE
The discovery of BACE1 inhibitors that reduce β-amyloid peptides in Alzheimer’s disease (AD) patients has been an encouraging development in the quest for a disease-modifying therapy. Kennedy and colleagues now report the discovery of verubecestat, a structurally unique, orally bioavailable small molecule that potently inhibits brain BACE1 activity resulting in a reduction in Aβ peptides in the cerebrospinal fluid of animals, healthy volunteers, and AD patients. No dose-limiting toxicities were observed in chronic animal toxicology studies or in phase 1 human studies, thus reducing safety concerns raised by previous reports of BACE inhibitors and BACE1 knockout mice.
Abstract
β-Amyloid (Aβ) peptides are thought to be critically involved in the etiology of Alzheimer’s disease (AD). The aspartyl protease β-site amyloid precursor protein cleaving enzyme 1 (BACE1) is required for the production of Aβ, and BACE1 inhibition is thus an attractive target for the treatment of AD. We show that verubecestat (MK-8931) is a potent, selective, structurally unique BACE1 inhibitor that reduced plasma, cerebrospinal fluid (CSF), and brain concentrations of Aβ40, Aβ42, and sAPPβ (a direct product of BACE1 enzymatic activity) after acute and chronic administration to rats and monkeys. Chronic treatment of rats and monkeys with verubecestat achieved exposures >40-fold higher than those being tested in clinical trials in AD patients yet did not elicit many of the adverse effects previously attributed to BACE inhibition, such as reduced nerve myelination, neurodegeneration, altered glucose homeostasis, or hepatotoxicity. Fur hypopigmentation was observed in rabbits and mice but not in monkeys. Single and multiple doses were generally well tolerated and produced reductions in Aβ40, Aβ42, and sAPPβ in the CSF of both healthy human subjects and AD patients. The human data were fit to an amyloid pathway model that provided insight into the Aβ pools affected by BACE1 inhibition and guided the choice of doses for subsequent clinical trials.

Brian Wang is a Futurist Thought Leader and a popular Science blogger with 1 million readers per month. His blog Nextbigfuture.com is ranked #1 Science News Blog. It covers many disruptive technology and trends including Space, Robotics, Artificial Intelligence, Medicine, Anti-aging Biotechnology, and Nanotechnology.
Known for identifying cutting edge technologies, he is currently a Co-Founder of a startup and fundraiser for high potential early-stage companies. He is the Head of Research for Allocations for deep technology investments and an Angel Investor at Space Angels.
A frequent speaker at corporations, he has been a TEDx speaker, a Singularity University speaker and guest at numerous interviews for radio and podcasts. He is open to public speaking and advising engagements.