Can helium bond with other elements to form a stable compound? Students attentive to Utah State University professor Alex Boldyrev’s introductory chemistry lectures would immediately respond “no.” And they’d be correct – if the scholars are standing on the Earth’s surface.
But all bets are off, if the students journey to the center of the Earth, à la Jules Verne’s Otto Lidenbrock or if they venture to one of the solar system’s large planets, such as Jupiter or Saturn.
“That’s because extremely high pressure, like that found at the Earth’s core or giant neighbors, completely alters helium’s chemistry,” says Boldyrev, faculty member in USU’s Department of Chemistry and Biochemistry.
Boldyrev’s colleagues confirmed computationally and experimentally that sodium, never an earthly comrade to helium, readily bonds with the standoffish gas under high pressure to form the curious Na2He compound. These findings were so unexpected, Boldyrev says, that he and colleagues struggled for more than two years to convince science reviewers and editors to publish their results.
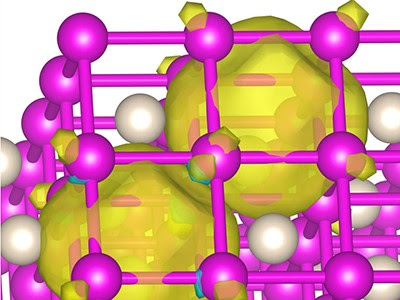
Image depicting structure formed by helium and sodium. On Earth, helium doesn’t bond with other elements. Yet, under high pressure, such as conditions found at the Earth’s core or on larger planets, it does, say USU researchers. Credit: Ivan Popov.
Nature Chemistry – A stable compound of helium and sodium at high pressure
Helium is generally understood to be chemically inert and this is due to its extremely stable closed-shell electronic configuration, zero electron affinity and an unsurpassed ionization potential. It is not known to form thermodynamically stable compounds, except a few inclusion compounds. Here, using the ab initio evolutionary algorithm USPEX and subsequent high-pressure synthesis in a diamond anvil cell, we report the discovery of a thermodynamically stable compound of helium and sodium, Na2He, which has a fluorite-type structure and is stable at pressures over 113 GPa. We show that the presence of He atoms causes strong electron localization and makes this material insulating. This phase is an electride, with electron pairs localized in interstices, forming eight-centre two-electron bonds within empty Na8 cubes. We also predict the existence of Na2HeO with a similar structure at pressures above 15 GPa.

Brian Wang is a Futurist Thought Leader and a popular Science blogger with 1 million readers per month. His blog Nextbigfuture.com is ranked #1 Science News Blog. It covers many disruptive technology and trends including Space, Robotics, Artificial Intelligence, Medicine, Anti-aging Biotechnology, and Nanotechnology.
Known for identifying cutting edge technologies, he is currently a Co-Founder of a startup and fundraiser for high potential early-stage companies. He is the Head of Research for Allocations for deep technology investments and an Angel Investor at Space Angels.
A frequent speaker at corporations, he has been a TEDx speaker, a Singularity University speaker and guest at numerous interviews for radio and podcasts. He is open to public speaking and advising engagements.