In the United States, extreme prematurity is the leading cause of infant morbidity and mortality, with over one-third of all infant deaths and one-half of cerebral palsy attributed to prematurity. Advances in neonatal intensive care have improved survival and pushed the limits of viability to 22 to 23 weeks of gestation. However, survival has been achieved with high associated rates of chronic lung disease and other complications of organ immaturity, particularly in infants born before 28 weeks. In fact, with earlier limits of viability, there are actually more total patients with severe complications of prematurity than there were a decade ago.
Currently, very premature infants, born at around 23 weeks of gestation, are placed in incubators and put on ventilators to help them breathe, but this can damage their lung development. In babies born preterm, the chance of survival at less than 23 weeks is close to zero, while at 23 weeks it is 15%, at 24 weeks 55% and at 25 weeks about 80%.
This new procedure has the possibility of enabling high probability healthy premature survival rates from 27 weeks to 22 or 23 weeks.
In the US, about 10 percent of babies are born prematurely. This means they were born before they reach 37 weeks of pregnancy. About 6 percent, or 30,000 of those births (0.6% of total births), are considered extremely premature, which means that they were born at or before the 28th week of pregnancy.
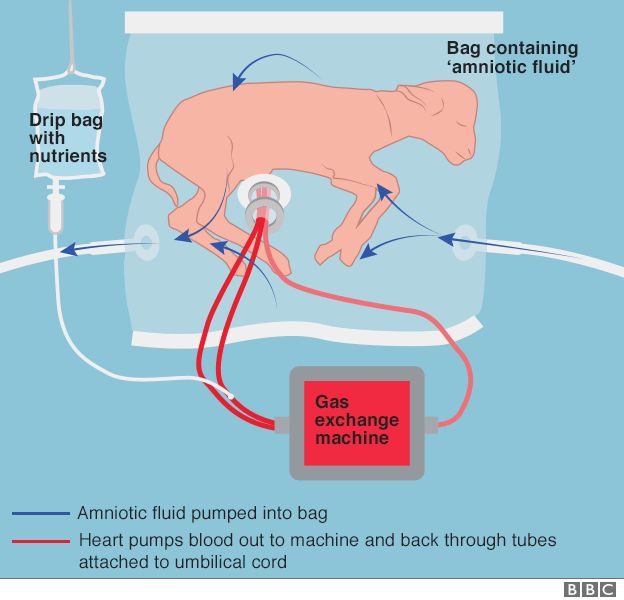
It provides everything the foetus needs to continue growing and maturing, including a nutrient-rich blood supply and a protective sac of amniotic fluid.
The approach might one day help premature human babies have a better chance of survival, experts hope.
Human trials may be possible in a few years, according to researchers.
First, more tests in animals are needed to check it is safe enough to progress.

Respiratory failure represents the most common and challenging problem, as gas exchange in critically preterm neonates is impaired by structural and functional immaturity of the lungs. This condition, known as bronchopulmonary dysplasia, is now understood to be related to an arrest in lung development secondary to premature transition from liquid to gas ventilation, explaining why even minimally invasive modes of neonatal ventilation have not reduced the incidence of bronchopulmonary dysplasia5. There is clearly an urgent need for a more physiologic approach to support the extreme premature infants.

The development of an ‘artificial placenta’ has been the subject of investigation for over 50 years with only limited success. The primary obstacles have been progressive circulatory failure due to preload or afterload imbalance imposed on the fetal heart by oxygenator resistance and pump-supported circuits, the use of open fluid incubators resulting in contamination and fetal sepsis and problems related to umbilical vascular access resulting in vascular spasm. To address these obstacles we have designed a system consisting of three main components, specifically, a pumpless arteriovenous circuit, a closed fluid environment with continuous fluid exchange and a new technique of umbilical vascular access. Here we demonstrate that extreme premature fetal lambs can be consistently supported in an extracorporeal device for up to 4 weeks without apparent physiologic derangement or organ failure. These results are superior to all previous attempts at extracorporeal support of the extreme premature fetus in both duration and physiologic well-being.
SOURCES- BBC News, Nature Communications

Brian Wang is a Futurist Thought Leader and a popular Science blogger with 1 million readers per month. His blog Nextbigfuture.com is ranked #1 Science News Blog. It covers many disruptive technology and trends including Space, Robotics, Artificial Intelligence, Medicine, Anti-aging Biotechnology, and Nanotechnology.
Known for identifying cutting edge technologies, he is currently a Co-Founder of a startup and fundraiser for high potential early-stage companies. He is the Head of Research for Allocations for deep technology investments and an Angel Investor at Space Angels.
A frequent speaker at corporations, he has been a TEDx speaker, a Singularity University speaker and guest at numerous interviews for radio and podcasts. He is open to public speaking and advising engagements.