The case for in situ resource utilisation for oxygen production on Mars by nonequilibrium plasmas
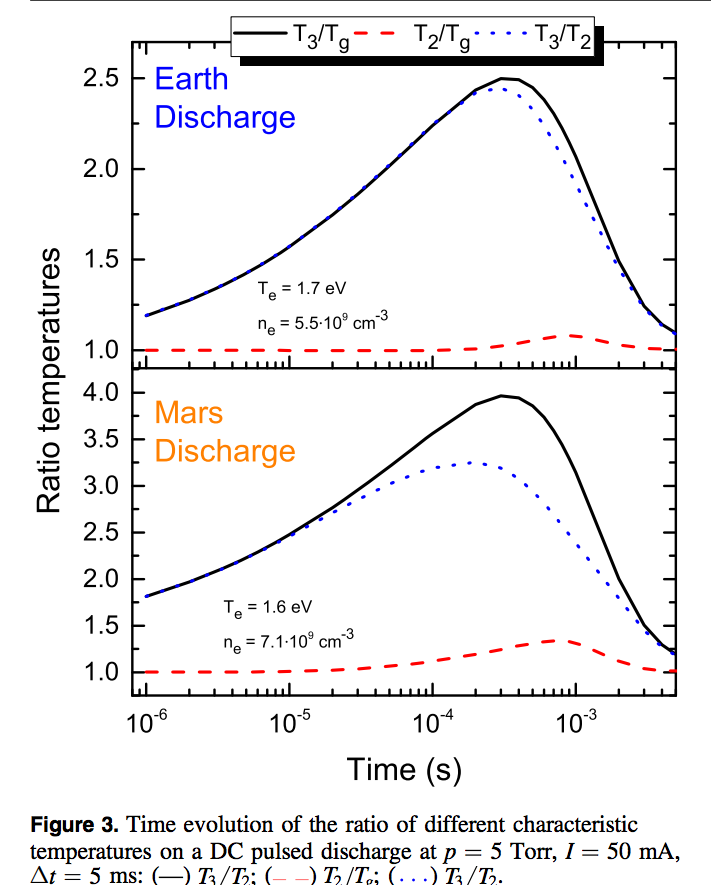
Herein, it is argued that Mars has nearly ideal conditions for CO2 decomposition by nonequilibrium plasmas. It is shown that the pressure and temperature ranges in the ~96% CO2 Martian atmosphere favour the vibrational excitation and subsequent up-pumping of the asymmetric stretching mode, which is believed to be a key factor for an efficient plasma dissociation, at the expense of the excitation of the other modes. Therefore, gas discharges operating at atmospheric pressure on Mars are extremely strong candidates to produce O2 efficiently from the locally available resources.
Local production of oxygen (O2) on Mars may help solve the problems of manufacturing fuel for coming back to Earth and of creating a breathable environment for a future outpost. In fact, the main component of the Martian atmosphere is carbon dioxide (CO2) (95.9%), with smaller percentages of Ar (1.9%), N2 (1.9%) and other gases. CO2 can be converted into O2 and carbon monoxide (CO), which were proposed to be used in a propellant mixture in rocket vehicles. Such in-situ resource utilisation (ISRU) will diminish the needs of additional launch or lander mass. Accordingly, it will minimise risks to the crew and mission, as well as reduce logistics, making it possible to increase the space-craft shielding and provide increased self-sufficiency. Moreover, it will reduce costs by demanding less launch vehicles to complete the mission.
Plasma reforming of CO2 on Earth is also a growing field of research, prompted by the problems of climate change and the production of solar fuels. Indeed, low-temperature plasmas constitute one of the best media for CO2 dissociation, both by direct electron impact and, especially, by transferring electron energy into vibrational excitation.
Plasma technologies for CO2 reforming on Earth are already competitive nowadays with solid oxide electroliser cells (SOEC). Therefore, our investigation evinces that a nonequilibrium plasma process can probably perform better than SOEC for O2 production on Mars, the technology proposed by the exciting MOXIE programme. In fact, while the efficiency of plasma dissociation of CO2 on Mars is likely to increase compared to that on Earth, as demonstrated in this work, the efficiency of solid oxide electrolysis is likely to decrease, because extra energy is necessary to heat the gas up to ∼1100 K and to compress it up to ∼1 atm. In addition, any estimation based on typical gas flows and CO2 conversion rates obtained on Earth points out that the throughput anticipated by the MOXIE experiment, of about 10 g per hour for a power of 300 W, is perfectly within the reach of an optimised plasma device.
A system needing only 150 to 200 Watts for 4 hours each 25-hour Mars day could produce 8 to 16 kilograms of oxygen. The International Space Station currently consumes oxygen in the range of 2 to 5 kilograms per day, so this would be enough to support a small settlement.

Brian Wang is a Futurist Thought Leader and a popular Science blogger with 1 million readers per month. His blog Nextbigfuture.com is ranked #1 Science News Blog. It covers many disruptive technology and trends including Space, Robotics, Artificial Intelligence, Medicine, Anti-aging Biotechnology, and Nanotechnology.
Known for identifying cutting edge technologies, he is currently a Co-Founder of a startup and fundraiser for high potential early-stage companies. He is the Head of Research for Allocations for deep technology investments and an Angel Investor at Space Angels.
A frequent speaker at corporations, he has been a TEDx speaker, a Singularity University speaker and guest at numerous interviews for radio and podcasts. He is open to public speaking and advising engagements.

Carbon Monoxide and Oxygen actually makes a decent rocket fuel; Not quite as good as Methane or Hydrogen and Oxygen, but plenty good for getting from point to point on Mars, or even going up to the moons and back. Nice thing is that you don’t have to mine water, you just need a port for the atmosphere to enter your factory.
So when Moon to Earth transportation is well established it would be another way of getting back to Earth via the moon base stop.
But there isn’t large supplies of CO2 on the moon, so you’d have to bring the fuel from Earth, in which case why not get a better fuel?
I think it might make sense for point to point transportation on Mars, and for a Mars to orbit and back “taxi”, But it’s a bit underperforming for flights from Mars to other planets.
What it mainly is, is a good option before you get the water mines set up, and for use exclusively on and around Mars.
Still think Zubrin’s nuclear thermal rocket using CO2 as reaction mass makes better sense for jumping around on Mars.
So, someone with more knowledge then I.. is there a formula that will tell you how much energy is required to convert X amount of C02 to O2? I’m curious what it would take to put something on Mars that actually starts to have an effect on the atmosphere in terms of lowering the CO2 and increasing the O2.
I know it’s SciFi, but someone akin to the Atmospheric Processing plants in Aliens.
Well, this report discusses the matter in some detail: http://www.uapress.arizona.edu/onlinebks/ResourcesNearEarthSpace/resources28.pdf
This is more focused on plasma reformation, the technology discussed above:
http://iopscience.iop.org/article/10.1088/1361-6595/aa8dcc
10 grams per hour at 300W is anticipated for the initial reactor to be placed on Mars in a 2020 experiment.
Pressure at ground level is about 0,7% Earth’s one on Mars, so you’d need much more atmosphere before willing a different one where CO2 would then be a trace gaz.
Best way to turn CO2 into oxygen is micro life (cyanobacteria and micro algea are much more efficient than plants) that you can have in a base.
But… You should think about Terraforming Mars only IF (a tremendous “IF”) there was a huge hidden amount of nitrogen to be discovered underground. Else, there’s no point in even trying…
Most probably it could be buried as ammonium salts because those were found on Ceres and Mars is frigid enough since a very long time without tectonic/volcanic activity to keep them mostly intact (if they are there).
Isotopic ratios indicate a huge loss of atmosphere more than 3,5 billions years ago but it’s possible a good fraction of it was already frozen underground. We need lots of data before even thinking to tinker Mars atmosphere… So let’s go there.
Anyway IF conditions are met you don’t Terraform a planet before having there a very significant population. Remember those big lava tubes discovered on Mars ? You could make them into huge Eartlike environment to accomodate on the order of 100 000 people long before any significant global modifications can be seen.
Send a robot processing plant ahead so when humans show up there will be plenty of O2 waiting.