All-solid-state batteries are new type of Li-ion battery that should be potentially safer and have higher energy densities.
Resistance at the electrode/solid electrolyte interface has been too high which prevents fast charging and discharging.
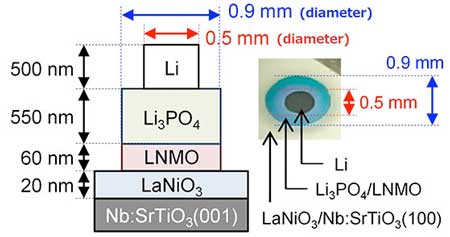
The resistance of this interface, verified using electrochemical impedance spectroscopy, was 7.6 Ωcm2, two orders of magnitude smaller than that of previous LMNO-based all-solid-state batteries and even smaller than that of liquid-electrolyte-based Li-ion batteries using LNMO. These batteries also displayed fast charging and discharging, managing to charge/discharge half the battery within just one second. Moreover, the cyclability of the battery was also excellent, showing no degradation in performance even after 100 charge/discharge cycles.
Solid-state Li batteries containing Li(Ni0.5Mn1.5)O4 as a 5 V-class positive electrode are expected to revolutionize mobile devices and electric vehicles. However, practical applications of such batteries are hampered by the high resistance at their solid electrolyte/electrode interfaces. Here, we achieved an extremely low electrolyte/electrode interface resistance of 7.6 Ω cm2 in solid-state Li batteries with Li(Ni0.5Mn1.5)O4. Furthermore, we observed spontaneous migration of Li ions from the solid electrolyte to the positive electrode after the formation of the electrolyte/electrode interface. Finally, we demonstrated stable fast charging and discharging of the solid-state Li batteries at a current density of 14 mA/cm2. These results provide a solid foundation to understand and fabricate low-resistance electrolyte/electrode interfaces.

Brian Wang is a Futurist Thought Leader and a popular Science blogger with 1 million readers per month. His blog Nextbigfuture.com is ranked #1 Science News Blog. It covers many disruptive technology and trends including Space, Robotics, Artificial Intelligence, Medicine, Anti-aging Biotechnology, and Nanotechnology.
Known for identifying cutting edge technologies, he is currently a Co-Founder of a startup and fundraiser for high potential early-stage companies. He is the Head of Research for Allocations for deep technology investments and an Angel Investor at Space Angels.
A frequent speaker at corporations, he has been a TEDx speaker, a Singularity University speaker and guest at numerous interviews for radio and podcasts. He is open to public speaking and advising engagements.

Interesting, it sounds like future batteries will be “printed” a layer at a time. I can imagine a 3D printer with thousands/millions of micro-nozzles that can print with any atomic matter needed. Cool. It would be even cooler if the batteries could be “grown”.
Mmmm… it does sound promising. It also sounds like practical (i.e. “with the right stuff working in concert to achieve functionally practical performance measures”) solid-state batteries having a non-liquid electrolyte will NECESSARILY be finely patterned at the nanoscale.
For example: the diagram above is like a layer-cake, with lithium blanketed by LiMnPO4, and that imbedded more or less in a non-ionic conductive “solid electrolyte”. Since the resistance of such a system COMES from having a diffusion-limited solid electrolyte (pushing UP the resistance thru the almost irreducible solid-matrix diffusion rate compared to a motile liquid), well the only choice is to make the diffusion barrier as thin as possible.
This in turn is exacted by heavily pumping up the nanoscale geometry parallelism. Put a billion nanobits in parallel, and tho’ each one has quite high resistance, the aggregate might actually have a fairly low bulk resistance. (Parallel resistors DIVIDE each other’s resistance in aggregate.)
But with this nanoscale parallelism comes fabrication complexity. LOTS of it. It might be possible to fabricate the billions of lithium nanowires and their coatings in bulk, “in vitro”. But then hooking them efficiently and geometrically tightly packed into the anode and cathode plate matrixes … in bulk … in production … by the millions … is another kettle of fish altogether.
Compare this to today’s lithium batteries.
Everything is done “bulk”. Vats of things are mixed, are milled, are blended under atmospheres of argon to keep them from burning up before assembly. They’re cast into forms, compacted by hydraulic presses, chamfered and trimmed to exacting standard, then assembled by robotic machines in parallel into the endless output stream of 18450 batteries (as an example) then used as commodity energy storage base units.
The “nanoscale” is a bulk phenomenon of the expanded graphitic carbon used as the anode matrix material. Its not at all precise.
AND THAT IS THE POINT.
Consider it.
[b]Goat[/b]Guy
I’m finally getting the sense that we’re on the brink of a major improvement in battery energy-density via solid-state batteries