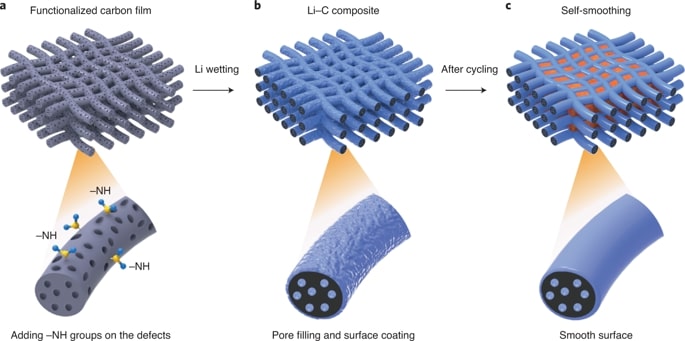
Despite considerable efforts to stabilize lithium metal anode structures and prevent dendrite formation, achieving long cycling life in high-energy batteries under realistic conditions remains extremely difficult due to a combination of complex failure modes that involve accelerated anode degradation and the depletion of electrolyte and lithium metal. Researchers report a self-smoothing lithium–carbon anode structure based on mesoporous carbon nanofibres, which, coupled with a lithium nickel–manganese–cobalt oxide cathode with a high nickel content, can lead to a cell-level energy density of 350–380 Wh per kg (counting all the active and inactive components) and a stable cycling life up to 200 cycles. These performances are achieved under the realistic conditions required for practical high-energy rechargeable lithium metal batteries: cathode loading over 4.0 mAh per cm^2, negative to positive electrode capacity ratio less than 2 and electrolyte weight to cathode capacity ratio less than 3 g per Ah. The high stability of our anode is due to the amine functionalization and the mesoporous carbon structures that favour smooth lithium deposition.
Nature Nanotechnology – Self-smoothing anode for achieving high-energy lithium metal batteries under realistic conditions

Brian Wang is a Futurist Thought Leader and a popular Science blogger with 1 million readers per month. His blog Nextbigfuture.com is ranked #1 Science News Blog. It covers many disruptive technology and trends including Space, Robotics, Artificial Intelligence, Medicine, Anti-aging Biotechnology, and Nanotechnology.
Known for identifying cutting edge technologies, he is currently a Co-Founder of a startup and fundraiser for high potential early-stage companies. He is the Head of Research for Allocations for deep technology investments and an Angel Investor at Space Angels.
A frequent speaker at corporations, he has been a TEDx speaker, a Singularity University speaker and guest at numerous interviews for radio and podcasts. He is open to public speaking and advising engagements.

Small and continuous baby steps, that’s how I believe innovation will proceed forward in batteries. Yet in a short five years, what we consider the latest well-performing battery will look like a Stone Age implement with what replaces it.
Yes, smoothing would be an issue. The ‘hope’ would be that using the mat-of-plaited-nanofibers, that the smoothing problem is something readily overcome.
Personally, I am beginning to wonder whether the lithium electrochemistry solid state battery thing is maturing to an eventual optimum of about 25% to 50% better than present-day Tesla cells.
Clearly “larger isn’t better”: it takes no crazy manufacturing to cobble together lithium cells 3× (each dimension, or 27× volumetrically) the size of say the 2170 Tesla (M3) format. And test them. Getting nearly the same 0.235 kWh/kg and 0.725 kWh/L specific energy densities.
The Problem remains the same: surface-area to volume, and electrochemistry charge-discharge heat dissipation. Ideally, the cells should be as large as possible, to minimize ratio of “can to guts”. More guts equals higher specific energy. However, lower and lower can:guts ratio also entrains the C-D heat better (bad), derating the higher charge/discharge specs of the cells.
Since e-vehicles are supposed to be super-peppy, that’d be bad.
So… Just saying,
GoatGuy ✓
What is stable cycling life? What would be the cycle life degrading to 80% capacity, could that be 500 cycles, 1000? Damned dendrites anyway…
i would sugest that smoothing might not be that ideal, as it smoothly locks out the annode over time (no chance of cracks in chemical surface waste deposite.. ea the way lithium bateries normally get old), also smooth surfaces have a smaller surface hence a less large chemical reaction area… just a theoretical observation.
Which is why I don’t think 100K is enough. But at 200K you’re probably not counting on much resale value. Besides that, there’s clearly a segment of the market willing to shell out for the best electric car available.
The original buyer of a new car does usually trade up by 100 000. But trading up implies a 2nd hand market.
You won’t get a second hand market if the battery is dead at that point.
Though as GG points out, this 200 cycles is early prototypes in the lab, not the stage at which they start selling it in cars.
It’s 70% better than Tesla batteries, giving about a 500 mile range, so 200 cycles is 100K miles. I agree that’s not long enough, but it’s not *too* far off from a point where by the time it’s worn out, you’re going to want to upgrade to newer tech anyway.
Being a bit more upbeat than I usually am (the Morning Coffee has Kicked In), I would venture that this is actually a fairly important development. Important from the point of view that it volumetrically improves the sequestration of lithium, thus improving the kWh/kg ratio of readily manufacturable batteries.
The 200 charge-discharge cycles seems a bit wan, but is the “first go” of development. Remember OLEDs? Oh, way back in 2004 or whatever?
Initially they were bright, complicated to make, and lasted only a few HOURS before sputtering out.
Eventually the boffins figured out how to protect the OLED material from H₂O vapor (and in manufacturing, from the host of barely detectable contaminants) that shortened its life. Today, you can purchase an LG OLED 42 inch screen at CostCo for less than $1,700. It is simply incredible… bright, contrasty, and supposed to last for a minimum of 10 years.
The point being… that this development needs to now be run thru quite a bit of boffinry to determine the causes for its ho-hum charge-discharge cycle lifetime, and overcome that. It would be nice to get above 2,000 CD cycles.
The interesting “however” is that Tesla (one imagines, not alone) determined that at 80% D and 80% C, their batteries last well above 1,500 cycles. More like 2,500, and probably closer to 3,000 on the average.
At 100 kWh = 360 miles × 80% = 288 miles × 2,500 = 720,000 miles …
I would say Good Enuf.
Just saying,
GoatGuy ✓
Yes… for what it is worth..
It’s not enough for phones or cars, sure, but depending on the cost, could be a viable replacement for non-rechargeable batteries.
Well, 200 cycles is only valuable for toys….