Guided by machine learning, chemists at the Department of Energy’s Oak Ridge National Laboratory designed a record-setting carbonaceous supercapacitor material that stores four times more energy than the best commercial material. A supercapacitor made with the new material could store more energy — improving regenerative brakes, power electronics and auxiliary power supplies.
This research has the potential to accelerate the development and optimization of carbon materials for supercapacitor applications. Although this breakthrough study used the best data at the time, scientists now have even more boundary data for training the machine learning model for the next study.
“By combining a data-driven method and our research experience, we created a carbon material with enhanced physicochemical and electrochemical properties that pushed the boundary of energy storage for carbon supercapacitors to the next level,” said chemist Tao Wang of ORNL and the University of Tennessee, Knoxville.
Commercial supercapacitors have two electrodes — an anode and cathode — that are separated and immersed in an electrolyte. Double electrical layers reversibly separate charges at the interface between the electrolyte and the carbon. The materials of choice for making electrodes for supercapacitors are porous carbons. The pores provide a large surface area for storing the electrostatic charge.
The ORNL-led study used machine learning, a type of artificial intelligence that learns from data to optimize outcomes, to guide the discovery of the superlative material. Runtong Pan, Musen Zhou and Jianzhong Wu from the University of California, Riverside, a FIRST partner university, built an artificial neural network model and trained it to set a clear goal: develop a “dream material” for energy delivery.
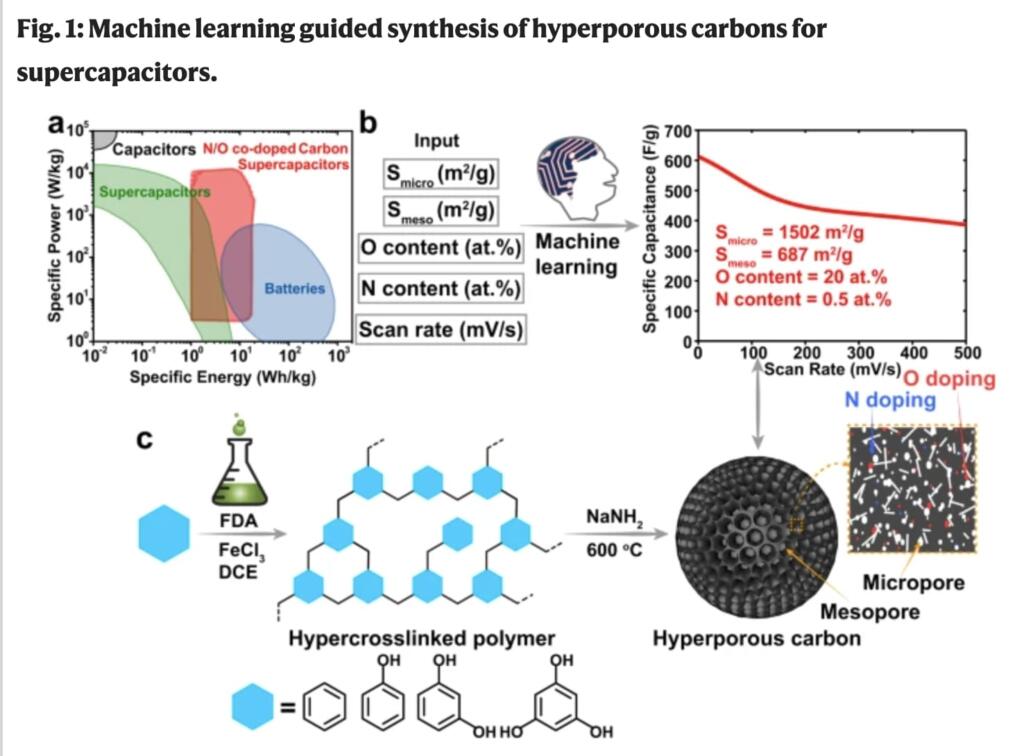
The model predicted that the highest capacitance for a carbon electrode would be 570 farads per gram if the carbon were co-doped with oxygen and nitrogen.
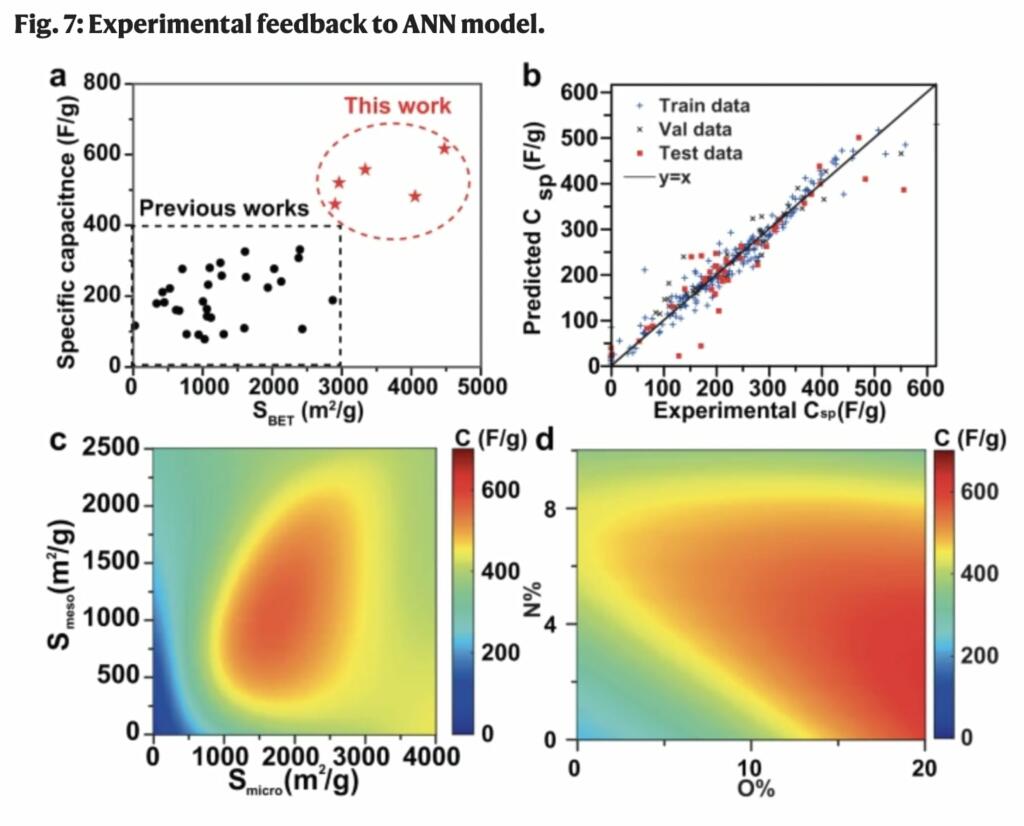
The synthesized material had a capacitance of 611 farads per gram — four times higher than a typical commercial material. Pseudocapacitance is storage of charge based on continuous, fast and reversible oxidation-reduction reactions at the surface of electrode materials. Pseudocapacitance from such reactions at the oxygen/nitrogen sites contributed to 25% of the overall capacitance. The material’s surface area was among the highest recorded for carbonaceous materials — more than 4,000 square meters per gram.
This success came quickly. The data-driven approach allowed Wang and Dai to achieve in three months what would have previously taken at least a year.
“We achieved the performance of carbon materials at the limit,” Wang said. “Without the goal that machine learning set, we would have kept optimizing materials through trial and error without knowing their limit.”
The key to success was achieving two kinds of pores — mesopores between 2 and 50 nanometers, or billionths of a meter, and micropores tinier than 2 nanometers. In experimental analyses, the chemists found that the combination of mesopore and micropores provided not only a high surface area for energy storage but also channels for electrolyte transport. Miaofang Chi and Zhennan Huang at the Center for Nanophase Materials Sciences, a DOE Office of Science user facility at ORNL, performed scanning transmission electron microscopy to characterize the mesopores, but the micropores were too small to see.
Microscopically, the material looks like a golf ball with deep dimples. The dimples represent mesopores, and the micropores exist in the material between the dimples.
Abstract
Porous carbons are the active materials of choice for supercapacitor applications because of their power capability, long-term cycle stability, and wide operating temperatures. However, the development of carbon active materials with improved physicochemical and electrochemical properties is generally carried out via time-consuming and cost-ineffective experimental processes. In this regard, machine-learning technology provides a data-driven approach to examine previously reported research works to find the critical features for developing ideal carbon materials for supercapacitors. Here, we report the design of a machine-learning-derived activation strategy that uses sodium amide and cross-linked polymer precursors to synthesize highly porous carbons (i.e., with specific surface areas > 4000 m2/g). Tuning the pore size and oxygen content of the carbonaceous materials, we report a highly porous carbon-base electrode with 0.7 mg/cm2 of electrode mass loading that exhibits a high specific capacitance of 610 F/g in 1 M H2SO4. This result approaches the specific capacitance of a porous carbon electrode predicted by the machine learning approach. We also investigate the charge storage mechanism and electrolyte transport properties via step potential electrochemical spectroscopy and quasielastic neutron scattering measurements.
Introduction
Aqueous supercapacitors are critical energy storage devices for applications that require high power density and long cycle lifetime, such as regenerative braking systems in electric vehicles, uninterruptible power supplies, and power levelers for electronics. With the fast development of supercapacitors, diverse materials including porous carbons, metal oxides/carbides/nitrides, and conductive polymers have been optimized to pursue a higher energy density in supercapacitors, among which porous carbons are still the primary and widely used active materials for commercial aqueous supercapacitors. The advantages of porous carbons for supercapacitors include power capability, long-term cycle stability, wide operating temperatures, and high Coulombic efficiencies. The basic energy storage mechanism of carbon supercapacitors is through an electrical double-layer capacitance (EDLC), derived from the reversible charge separation at the interface of the electrolyte with the carbon surface. The large surface area and appropriate pore structure of carbon supercapacitors are crucial to provide a large interfacial area for a large EDLC as well as a fast ionic mobility path for high charge/discharge rates. However, the experimental exploration for appropriate pore structures necessitates the formulation of intricate synthetic strategies, and the execution of time-intensive experimental processes including tedious synthesis of various carbon materials, characterization of pore structures, electrochemical tests, and data analysis. All of which, in the absence of a definitive guideline, become a time-consuming endeavor. Recently, the development of machine-learning technology provides a data-driven approach to learning from extensive previously reported works and points out the critical features of an ideal supercapacitor device with carbon-based electrodes25,26. The maximum value (250 F/g at a scan rate of 1 mV/s) for pristine carbon electrodes in 6 M KOH electrolyte has been predicted by the data-driven machine learning model based on the artificial neural network (ANN), which is achieved when the porous carbon has the specific surface areas of micropores (pores with widths not exceeding about 2 nm) and mesopores (pores with width between 2 and 50 nm) at 700 and 300 m2/g, respectively26. However, the low specific capacitance and bulk density of porous carbons limit their volumetric performance27. A heteroatom doped carbon surface with electro-active species such as N/O sites, and metal/metal oxide particles provides pseudocapacitance through quick and reversible faradic reactions, which contributes to high overall specific gravimetric and volumetric capacitance values28,29,30,31,32,33,34,35. Compared to the pristine carbon, the ANN predicted maximum value for N/O-doped carbons up to 570 F/g at a scan rate of 1 mV/s in base electrolyte (viz., 6 M KOH) occurs at the larger specific surface areas from micropores and mesopores (1400 and 1000 m2/g, respectively)26,36. The cyclic stability of carbon-based supercapacitors could be investigated by cycling or voltage holding tests9. According to machine learning aided cyclic stability prediction, enhanced cyclic stability could be achieved after complete activation of the electrodes and the construction of three dimensional porous structures37. Based on the guideline provided by ANN machine-learning results, the construction of highly porous carbon with appropriate pore structure and heteroatom doping is promising to achieve a high energy density for supercapacitors.
The theoretical upper limit of the specific surface area for carbonaceous materials was 2630 m2/g, which was calculated from infinite single-layer defectless graphene38. A higher surface area up to 7745 m2/g could be achieved by dividing the infinite layer into isolated six-membered rings, which maximizes the number of exposed ring faces and edges for graphitic carbons with sp2 hybridization39. Besides the graphitic carbons, the other way to expose more ring faces and edges is to avoid the alignment of six-membered rings by connecting six-membered rings with sp3-hybridized carbon or forming an amorphous structure

Brian Wang is a Futurist Thought Leader and a popular Science blogger with 1 million readers per month. His blog Nextbigfuture.com is ranked #1 Science News Blog. It covers many disruptive technology and trends including Space, Robotics, Artificial Intelligence, Medicine, Anti-aging Biotechnology, and Nanotechnology.
Known for identifying cutting edge technologies, he is currently a Co-Founder of a startup and fundraiser for high potential early-stage companies. He is the Head of Research for Allocations for deep technology investments and an Angel Investor at Space Angels.
A frequent speaker at corporations, he has been a TEDx speaker, a Singularity University speaker and guest at numerous interviews for radio and podcasts. He is open to public speaking and advising engagements.


The application to batteries or cars isn’t the big point here. ML is being used for better science. More complete understanding of problem/solution boundaries. Faster exploration of solution spaces. Doing science better might catch on.
Hi Phil,
Agreed the main point is that AI is helping to figure what would be optimal and guiding people to actually make the more optimal things.
[ Congratulations for that impressive improvement.
‘A higher surface area up to 7745 m2/g could be achieved by dividing the infinite layer into isolated six-membered rings’
this work ~3000-4500m2/g (~600F/g, 600F at ~2.5V results to ~0.5Wh,~1800J) =~0.38-0.58 from this (theoretical limit?) with isolated six-membered rings (volumes/g comparable?)
What if AGI approaches to 4/5 of the theoretical limit of all (more likely yet non-optimized, vs. e.g. electrical motors/generators or (solar, machining) inverters with possibly ~gt97% efficiency) areas of scientific research and development, with suggestions for applied science methods/devices?
Given AGI will be faster than all human research/testing&evaluating/production groups with enabling progress in materials&structuring&organizing optimization, what is going to be next while approaching to physics/theoretical (best suggestions for extending general theoretical understanding/coherency within physics&science) limits? ]
1-2% by weight of this super-capacitor combined with 98-99% battery would make for much less battery degradation from regenerative braking with little impact on range.
Liquid hydrocarbons have the highest energy density. Falcon 9 rocket fuel is kerosene. Smart people fuel their vehicle with liquid hydrocarbons.
no thanks.
I like leaving home with a “full tank”.
I like peppy acceleration.
I like virtually zero maintenance.
I like no noise.
I like zero exhaust fumes.
I like giving the finger to OPEC.
But whatever, you do you.
Not when they warm the planet.
Except Co2 isn’t an anthropogenic warming gas, all but one of earth’s ice ages was preceded by higher than todays levels of co2. You can’t be a runaway greenhouse gas if you precede ice ages. There is a limit to how much thermal radiation co2 can absorb and we’ve pretty much been there for a long while.
You should be concerned over cloud coverage, water vapor has a bigger effect and has been increasing.
However, the other factors Derek mentioned are good reasons to switch when the total cost of ownership goes down.
We may even already be past that point, Tesla’s model 3 is likely there, we just don’t know it yet due to the fact they haven’t been around long enough to give us the total lifetime cost of ownership.
Even if Tesla’s model 3 isn’t there, their next planned model starting in the $25,000 price range should be.
The first thing you should ask yourself is why you are paying attention to websites not associated with climate scientists? The second question you should ask yourself is why would cloud cover or water vapor be increasing? The well known lag between temps and CO2 concentration historically that is exactly what you would expect when there is feedback between the two. No surprises there. Stick to reputable Science rather than dodgy websites.
Apparently some process is causing oceans to warm which leads to outgassing and this outgassing has no considerable effect on temperature as the signal is swamped by a background of water vapor.
Feedback is a non-proven hypothesis only.
The lag demonstrates CO2 is not the cause.
This sudden rise in CO2 happens in every interglacial, right before a drop in temperature, regardless of the ppm CO2.
“Apparently some process is causing oceans to warm”.
If you want to stick your head in the sand and ignore the Science, so be it. Feel free to think the world is flat and we were created 8000 years ago. I’m not going to bother trying to pull you out.
Great Marcus, because blaming a delayed effect for being the cause is not plausible science. Not remotely.
You’ll learn eventually.
[ including 25-45% ‘loss’ within conversion, liquid hydrocarbons (methanol, compressed CH4, electrolysis to H2, O2 ~0.6-0.85) can be synthesized from solar energy input
solar(wind,hydro),geothermal(, extraterrestrial nuclear) electricity – battery – electric car motor =~0.75 (prospectively ~0.8x)
solar(wind,hydro),geothermal(, extraterrestrial nuclear) electricity – electrolysis – fuel cell – electric car motor =~0.41 (prospective ~0.55) ]